filmov
tv
13.1 Solution Formation and Solubility | General Chemistry
Показать описание
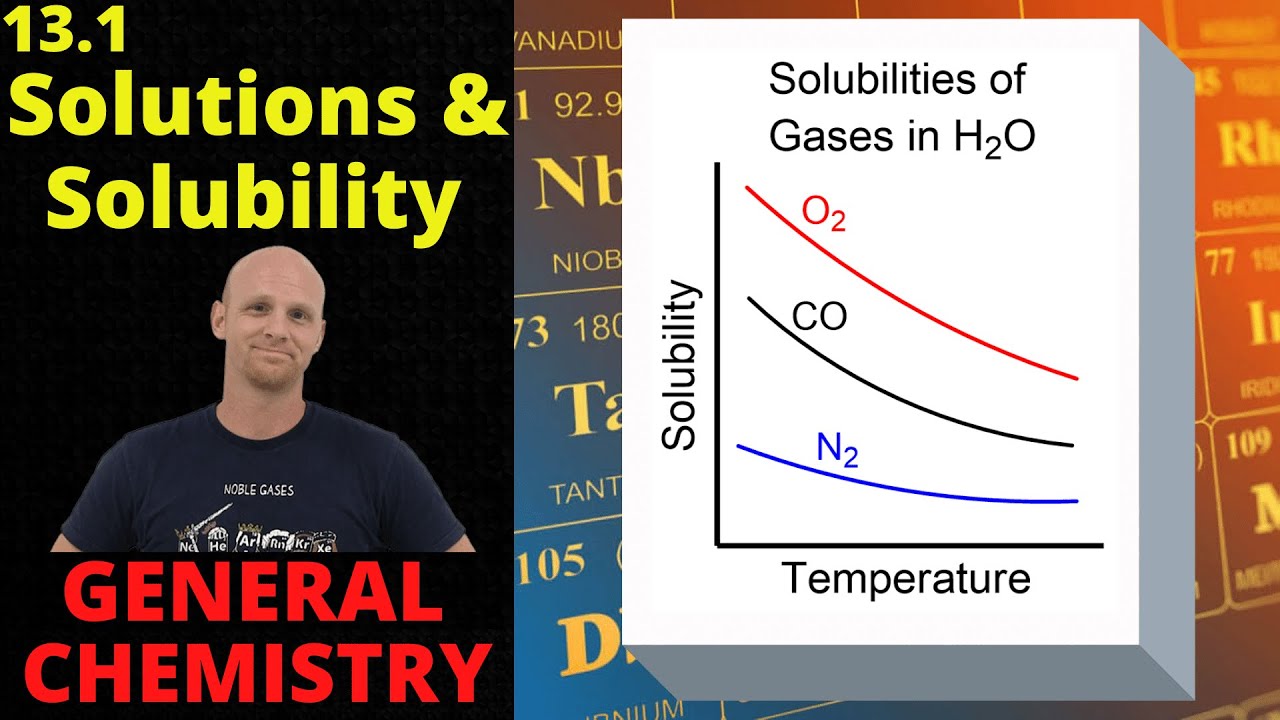
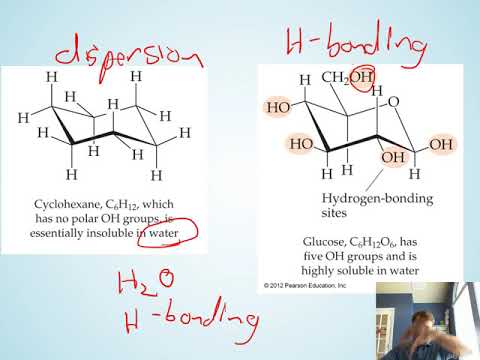
Chad provides an introductory lesson on Solutions. The lesson begins with a description of the 3 steps of the solution process and the energetics involved. This is then used to explain why some substances mix while others do not. The principle of "like dissolves like" is introduced and a variety of solutions vocabulary terms are defined including miscible, immiscible, saturated, unsaturated, supersaturated, and colloids. The lesson concludes with a discussion on some of the factors affecting the solubility of gases and ionic solids. This includes the affect of temperature and pressure on the solubility of gases including Henry's Law, and the affect of temperature on the solubility of ionic compounds in water.
00:00 Lesson Introduction
00:38 The Process of Solution Formation
06:40 Miscible vs Immiscible
07:34 Saturated, Unsaturated, & Supersaturated
09:16 Colloids
10:59 Solubility of Gases & Henry's Law
14:49 Solubility of Ionic Compounds in Water
00:00 Lesson Introduction
00:38 The Process of Solution Formation
06:40 Miscible vs Immiscible
07:34 Saturated, Unsaturated, & Supersaturated
09:16 Colloids
10:59 Solubility of Gases & Henry's Law
14:49 Solubility of Ionic Compounds in Water
13.1 Solution Formation and Solubility | General Chemistry
Solute, Solvent, & Solution - Solubility Chemistry
Complex Ion Equilibria - Stepwise Formation Constant Kf & Ksp Molar Solubility Problems
[H2 Chemistry] 2021 Topic 13 Solubility Equilibria In-Class Exercise 1 Review
Chapter 13 Section 2: Types of Solutions and Solubility
Chapter 13 - Properties of Solutions: Part 1 of 11
Solution Formation & Solubility 1718
[H2 Chemistry] 2021 Topic 13 Solubility Equilibria
Ksp - Molar Solubility, Ice Tables, & Common Ion Effect
Solubility vs Concentration - Basic Introduction, Saturated Unsaturated and Supersaturated Solutions
Energetics of Solution Formation
13 - Solutions and Colligative Properties
Solutions Overview and Types
Introduction to solubility equilibria | Equilibrium | AP Chemistry | Khan Academy
Solubility Rules and How to Use a Solubility Table
Worked example: Calculating solubility from Kₛₚ | Equilibrium | AP Chemistry | Khan Academy
Solutions and Solubility
Chapter 13 Part 2 - thermodynamics of forming a solution, and solubility with temperature
AP Chemistry Solution Formation, Spontaneity and Factors Affecting Solubility
Chapter 13:5 Solubility Curve
TRICKS you can do in SCIENTIFIC CALCULATORS🔥#viral #shorts
Video 13/17 Solutions, Table G, Table F, Solubility of Gases vs. Solids. Chemistry.Regents360
sulphuric acid #shorts
Chapter 13 - 2 Solubility Examples
Комментарии
 0:16:17
0:16:17
 0:16:09
0:16:09
 0:13:50
0:13:50
![[H2 Chemistry] 2021](https://i.ytimg.com/vi/zqq07Zeu4uY/hqdefault.jpg) 0:19:08
0:19:08
 0:09:59
0:09:59
 0:09:18
0:09:18
 0:16:15
0:16:15
![[H2 Chemistry] 2021](https://i.ytimg.com/vi/SoBiUMcTgpo/hqdefault.jpg) 1:27:45
1:27:45
 0:41:52
0:41:52
 0:13:20
0:13:20
 0:02:28
0:02:28
 0:40:37
0:40:37
 0:12:16
0:12:16
 0:08:17
0:08:17
 0:07:35
0:07:35
 0:04:52
0:04:52
 0:15:25
0:15:25
 0:25:03
0:25:03
 0:12:38
0:12:38
 0:17:19
0:17:19
 0:00:25
0:00:25
 0:31:25
0:31:25
 0:00:17
0:00:17
 0:10:31
0:10:31