filmov
tv
What is Nuclear Decay? | Daily Dose of Science

Показать описание
#palebluethoughts #dailydoseofscience #science
Alright, so you know how atoms are like these tiny building blocks that make up everything around us? Well, sometimes these atoms aren't stable and they need to change to become more stable. This process of change in unstable atoms is what we call nuclear decay. Imagine an unstable atom as a wobbly chair. It wants to steady itself, so it undergoes nuclear decay. During this process, the atom gets rid of excess energy or particles by emitting them, just like the wobbly chair might shed some extra bits to balance itself.
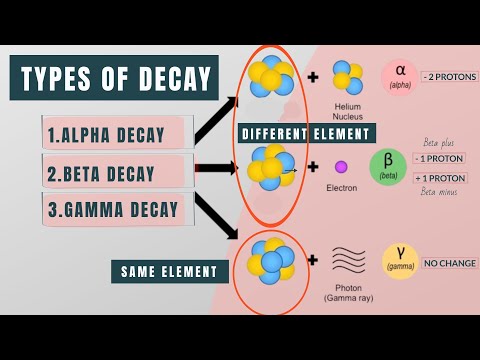
Now, there are a few ways this decay can happen. One is called alpha decay, where an atom releases an alpha particle made of two protons and two neutrons. Then there's beta decay. Here, a neutron in the atom changes into a proton or vice versa, and an electron or positron is emitted. This helps the atom achieve a more balanced state. Lastly, there's gamma decay. After alpha or beta decay, the atom might still have some extra energy hanging around. To get rid of that energy, it releases gamma rays, which are like super high-energy light particles.
Alright, so you know how atoms are like these tiny building blocks that make up everything around us? Well, sometimes these atoms aren't stable and they need to change to become more stable. This process of change in unstable atoms is what we call nuclear decay. Imagine an unstable atom as a wobbly chair. It wants to steady itself, so it undergoes nuclear decay. During this process, the atom gets rid of excess energy or particles by emitting them, just like the wobbly chair might shed some extra bits to balance itself.
Now, there are a few ways this decay can happen. One is called alpha decay, where an atom releases an alpha particle made of two protons and two neutrons. Then there's beta decay. Here, a neutron in the atom changes into a proton or vice versa, and an electron or positron is emitted. This helps the atom achieve a more balanced state. Lastly, there's gamma decay. After alpha or beta decay, the atom might still have some extra energy hanging around. To get rid of that energy, it releases gamma rays, which are like super high-energy light particles.
 0:04:54
0:04:54
 0:04:20
0:04:20
 0:04:24
0:04:24
 0:08:08
0:08:08
 0:00:15
0:00:15
 0:04:37
0:04:37
 0:08:02
0:08:02
 0:00:48
0:00:48
 1:09:32
1:09:32
 0:14:12
0:14:12
 0:08:08
0:08:08
 0:03:01
0:03:01
 0:00:59
0:00:59
 0:13:50
0:13:50
 0:09:58
0:09:58
 0:06:27
0:06:27
 0:00:30
0:00:30
 0:12:28
0:12:28
 0:03:37
0:03:37
 0:00:32
0:00:32
 0:06:41
0:06:41
 0:08:29
0:08:29
 0:04:42
0:04:42
 0:00:36
0:00:36