filmov
tv
How Radioactive Decay Works? Explained With Animation

Показать описание
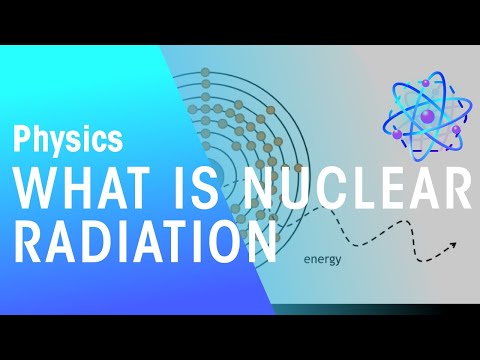
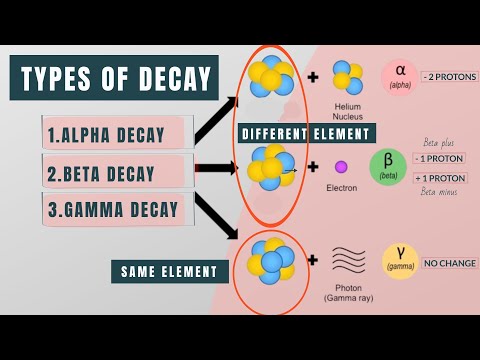
Radioactive decay is a natural and random process by which the nucleus of an unstable atom transforms into a more stable configuration. Radioactive decay was a puzzle for classical physics because it seemingly violated the principle of conserving energy. Quantum mechanics, however, introduces the element of randomness, allowing slightly unstable atomic nuclei to spontaneously transform into something more stable.
Even for the most unstable nuclei, predicting when a single atom will decay is impossible. This unpredictability is due to the strong nuclear force that holds protons and neutrons within the nucleus. While an atom might prefer a different combination of protons and neutrons, reaching that state requires removing or altering some components, which costs energy.
Each radioactive material has a characteristic half-life, which is the time it takes for half of a sample of that material to undergo radioactive decay. Different radionuclides have different half-lives, ranging from fractions of a second to millions of years. While we can't predict precisely when a particular nucleus will decay, we can estimate how long it will take for a given quantity of radioactive material to decay. This predictability is based on statistical principles and many atoms involved.
Despite this unpredictability at the atomic level, we can make statistical predictions for populations of atoms. This concept underlies the notion of a half-life, which specifies the time it takes for half the mass of a radioactive substance to undergo decay.
Editor: Team 121 Creators
Presenter: Sidhart Viyapu
Project Head: Rajkumar Shukla
Production: World Of Science Media
©2023, World Of Science (WOS) Media. All Rights Reserved
Even for the most unstable nuclei, predicting when a single atom will decay is impossible. This unpredictability is due to the strong nuclear force that holds protons and neutrons within the nucleus. While an atom might prefer a different combination of protons and neutrons, reaching that state requires removing or altering some components, which costs energy.
Each radioactive material has a characteristic half-life, which is the time it takes for half of a sample of that material to undergo radioactive decay. Different radionuclides have different half-lives, ranging from fractions of a second to millions of years. While we can't predict precisely when a particular nucleus will decay, we can estimate how long it will take for a given quantity of radioactive material to decay. This predictability is based on statistical principles and many atoms involved.
Despite this unpredictability at the atomic level, we can make statistical predictions for populations of atoms. This concept underlies the notion of a half-life, which specifies the time it takes for half the mass of a radioactive substance to undergo decay.
Editor: Team 121 Creators
Presenter: Sidhart Viyapu
Project Head: Rajkumar Shukla
Production: World Of Science Media
©2023, World Of Science (WOS) Media. All Rights Reserved
Комментарии
 0:00:59
0:00:59
 0:04:54
0:04:54
 0:06:27
0:06:27
 0:08:08
0:08:08
 0:04:54
0:04:54
 0:13:50
0:13:50
 0:25:07
0:25:07
 0:01:08
0:01:08
 0:12:28
0:12:28
 0:04:37
0:04:37
 0:14:12
0:14:12
 0:04:03
0:04:03
 0:03:01
0:03:01
 0:06:07
0:06:07
 0:08:08
0:08:08
 0:01:00
0:01:00
 0:07:42
0:07:42
 0:04:21
0:04:21
 0:04:16
0:04:16
 0:03:59
0:03:59
 0:02:45
0:02:45
 0:14:47
0:14:47
 0:11:48
0:11:48
 0:09:23
0:09:23