filmov
tv
Equation for NaOH + H2O (Sodium hydroxide + Water)

Показать описание
In this video we will describe the equation NaOH + H2O and write what happens when NaOH is dissolved in water.
When NaOH is dissolved in H2O (water) it will dissociate (dissolve) into Na+ and OH- ions. To show that they are dissolved in water we can write (aq) after each. The (aq) shows that they are aqueous – dissolved in water.
The equation for NaOH (Sodium hydroxide) and H2O sometimes isn’t considered a chemical reaction since it is easy to change the Na+ and OH- back to NaOH (just let the H2O evaporate). At the same time, the NaOH is a very different substance than Na+ and OH-.
If you need to know how to balance chemical reactions, see my complete tutorial on balancing all types of chemical equations:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
When NaOH is dissolved in H2O (water) it will dissociate (dissolve) into Na+ and OH- ions. To show that they are dissolved in water we can write (aq) after each. The (aq) shows that they are aqueous – dissolved in water.
The equation for NaOH (Sodium hydroxide) and H2O sometimes isn’t considered a chemical reaction since it is easy to change the Na+ and OH- back to NaOH (just let the H2O evaporate). At the same time, the NaOH is a very different substance than Na+ and OH-.
If you need to know how to balance chemical reactions, see my complete tutorial on balancing all types of chemical equations:
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Equation for NaOH + H2O (Sodium hydroxide + Water)
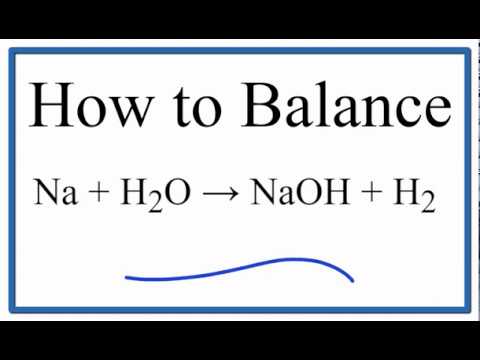
How to Balance Na + H2O = NaOH + H2 (Sodium plus Water)
How to Balance NaOH + H2SO4 = Na2SO4 + H2O
How to Write the Net Ionic Equation for NaOH + H2O
How to Balance Na2O + H2O = NaOH (Sodium Oxide plus Oxygen Gas)
Balancing Equation Na + H2O = NaOH + H2 (and Type of Reaction)
How to Balance Na + H2O = NaOH + H2 (Sodium plus Water)
How to Balance Al + NaOH + H2O = NaAlO2 + H2
Na+H2O ... Reaction between Sodium and Water
How to Balance Na2O2 + H2O = NaOH + O2 (Sodium peroxide + HOT Water)
Exprement with Potassium Permanganate & Sodium hydroxide|| #experiment #shorts #viral #ytshort...
How to Balance NaH + H2O = NaOH + H2 (Sodium hydride + Water)
How to Balance NaOH + H3PO4 = Na3PO4 + H2O (Sodium hydroxide and Phosphoric acid)
Sodium and potassium vs water
Balancing chemical equation? sodium + water =? 🧪🧪🧫🧫
How to Balance HNO3 + NaOH = NaNO3 + H2O (Nitric Acid plus Sodium Hydroxide)
Simple and easy science experiments.Electrolysis of water with NaCl.#shorts #diy #experiment #dwe .
How to balance: NaOH + H2SO4→ Na2SO4+ H2O
Type of Reaction for CH3COOH + NaOH = NaCH3COO + H2O
Explosion theory of Sodium hydroxide and water #chemistry #science #sodiumhydroxide
How to Balance NaOH + Cl2 = NaCl + NaClO + H2O (Dilute Sodium hydroxide + Chlorine gas)
NaOH in Water | NaOH pellets in Water #shorts
REACTION OF NAOH AND WATER | EXOTHERMIC REACTION #SCIENCE #ICSCE_CHEMISTRY
How to balance NaOH + HCl → NaCl + H2O
Комментарии
 0:01:30
0:01:30
 0:02:02
0:02:02
 0:02:37
0:02:37
 0:01:50
0:01:50
 0:01:00
0:01:00
 0:02:07
0:02:07
 0:02:42
0:02:42
 0:02:59
0:02:59
 0:02:23
0:02:23
 0:02:51
0:02:51
 0:00:28
0:00:28
 0:01:00
0:01:00
 0:02:34
0:02:34
 0:00:24
0:00:24
 0:01:00
0:01:00
 0:01:13
0:01:13
 0:00:15
0:00:15
 0:02:37
0:02:37
 0:02:12
0:02:12
 0:00:54
0:00:54
 0:01:58
0:01:58
 0:00:28
0:00:28
 0:00:56
0:00:56
 0:03:25
0:03:25