filmov
tv
Atomic Mass, Mass Number and Unified Atomic Mass Unit
Показать описание
----------------------------------------------------
An easy to understand chemistry tutorial, aimed to meet the GCSE (high school grade 9/10) higher-tier requirements in Chemistry.
Atoms are really really small, and consequently their mass is really small. This means that if we use units like 'grams' we get really unruly numbers that are hard to grapple with. Instead, we use a system called the unified atomic mass unit (or u for short).
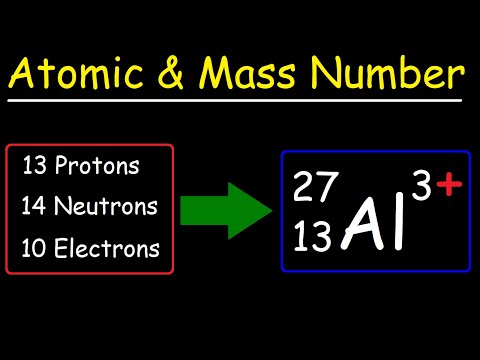
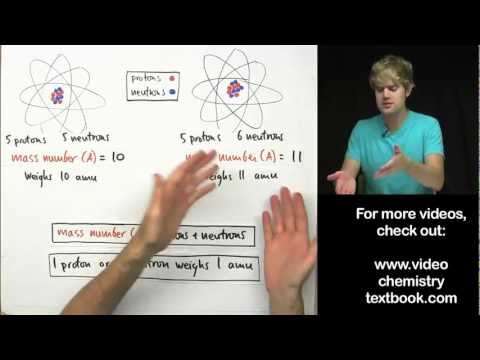
The atomic mass of an atom, is its actual mass - this is in contrast to mass number, which is the number of subatomic particles in the atom's nucleus that gives the atom its mass.
Using the unified atomic mass unit, we are able to compare the atomic masses of different atoms without having to use large numbers - and that's usually what we want to do: to be able to work out if one atom is heavier than another.
The atomic mass of carbon-12 is 12 u - and that's not a coincidence. 1 unit of the unified atomic mass system is defined as being 1/12th of the mass of carbon-12. That is, we use carbon-12 as the standard by which we compare all other atoms in the universe.
The video has a few examples, and gets you to decide if you think the atomic mass is going to be higher or lower than carbon-12's atomic mass. NB: although the atomic mass of an atom is not the same as its mass number, it is usually a very similar number.
You can turn the subtitles / captions on and off as you please, using the button in the bottom right hand corner.
----------------------------------------------------
An easy to understand chemistry tutorial, aimed to meet the GCSE (high school grade 9/10) higher-tier requirements in Chemistry.
Atoms are really really small, and consequently their mass is really small. This means that if we use units like 'grams' we get really unruly numbers that are hard to grapple with. Instead, we use a system called the unified atomic mass unit (or u for short).
The atomic mass of an atom, is its actual mass - this is in contrast to mass number, which is the number of subatomic particles in the atom's nucleus that gives the atom its mass.
Using the unified atomic mass unit, we are able to compare the atomic masses of different atoms without having to use large numbers - and that's usually what we want to do: to be able to work out if one atom is heavier than another.
The atomic mass of carbon-12 is 12 u - and that's not a coincidence. 1 unit of the unified atomic mass system is defined as being 1/12th of the mass of carbon-12. That is, we use carbon-12 as the standard by which we compare all other atoms in the universe.
The video has a few examples, and gets you to decide if you think the atomic mass is going to be higher or lower than carbon-12's atomic mass. NB: although the atomic mass of an atom is not the same as its mass number, it is usually a very similar number.
You can turn the subtitles / captions on and off as you please, using the button in the bottom right hand corner.
----------------------------------------------------
Комментарии
 0:11:41
0:11:41
 0:06:38
0:06:38
 0:08:57
0:08:57
 0:03:23
0:03:23
 0:05:04
0:05:04
 0:02:24
0:02:24
 0:03:50
0:03:50
 0:04:35
0:04:35
 0:08:10
0:08:10
 0:03:22
0:03:22
 0:06:13
0:06:13
 0:08:26
0:08:26
 0:05:53
0:05:53
 0:06:27
0:06:27
 0:02:48
0:02:48
 0:02:05
0:02:05
 0:13:12
0:13:12
 0:09:44
0:09:44
 0:02:41
0:02:41
 0:04:23
0:04:23
 0:13:46
0:13:46
 0:13:19
0:13:19
 0:07:01
0:07:01
 0:00:59
0:00:59