filmov
tv
estimating anticipated human dose for a preclinical drug

Показать описание
As a lead series advances through drug discovery and generates a compound with adequate preliminary safety, efficacy, and PK properties, researchers will formally estimate an anticipated human dose (AHD). The anticipated human dose will be driven by the efficacy (the PD) of the compound and the PK of the compound. The anticipated human dose is the amount of drug that is expected to be necessary to provide efficacious exposure of the drug for a patient. The anticipated human dose checks the feasibility of a drug dosage with regard to potency and efficacy and to ensure alignment with physicochemical properties like solubility. Efficacy drives the minimum dose – the minimal amount of drug to achieve a desired therapeutic benefit. PK affects the size of the dose as well as the dosing frequency. The dose amounts and frequency predictions are generated from the preclinical data gathered up to that point in the discovery program. How about safety? Safety is critically important, but the anticipated human dose is typically estimated before all the preclinical safety data are complete. The anticipated human dose is not the same as the first-in-human dose for phase I clinical trials. The first-in-human dose includes safety considerations.
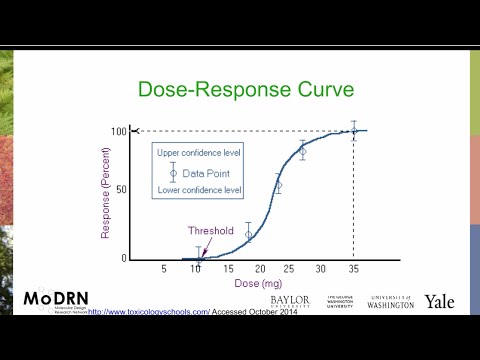
Here is a graph that allows visualization of the anticipated human dose prediction. At the time of the prediction, the discovery team will understand the degree of target engagement required to achieve a desirable degree of effect. Target engagement is driven by the unbound concentration (Cu) of the drug, which is the product of Cp and unbound fraction. So, target engagement arises from a threshold Cp value, and the threshold required Cp value can be extrapolated into a corresponding dose for the patient. Keep in mind that, at this stage, human PK is undetermined, so the human dose predictions involve estimates based on available animal PK data.
Evidence that allows prediction of the anticipated human dose starts with in vitro methods. In vitro methods are relevant to both the efficacy and PK of a compound. In vitro ADME studies provide preliminary insight into a compound’s pharmacokinetic properties, including clearance, bioavailability, and unbound fraction in plasma. In vitro binding, inhibition, and cellular assays provide early insight into compound concentrations required to provide target engagement and biological pathway modulation. Preclinical in vivo studies confirm the ADME data and allow confirmation and determination of all the PK parameters. Researchers also confirm in vivo efficacy in an animal disease model. Finally, PK/PD studies provide insight into how the dosing regimen may need to be optimized. One challenge with these data is that the in vivo studies are in preclinical species, not in humans. Based on decades of experience transitioning compounds from preclinical research and into clinical trials, scientists can provide an anticipated human dose. The anticipated dose can then be checked against properties such as solubility of the compound and projected formulations to ensure all the plans are feasible.
 0:04:57
0:04:57
 0:05:25
0:05:25
 0:17:32
0:17:32
 0:28:51
0:28:51
 1:19:13
1:19:13
 0:08:16
0:08:16
 1:09:08
1:09:08
 1:07:16
1:07:16
 0:05:26
0:05:26
 0:18:06
0:18:06
 0:49:06
0:49:06
 0:06:58
0:06:58
 0:00:39
0:00:39
 0:56:28
0:56:28
 0:54:24
0:54:24
 0:08:35
0:08:35
 0:20:35
0:20:35
 0:25:55
0:25:55
 0:41:34
0:41:34
 0:28:35
0:28:35
 0:29:33
0:29:33
 0:14:30
0:14:30
 0:02:01
0:02:01
 0:09:07
0:09:07