filmov
tv
Calculate Faraday's Constant - Electroplating 002

Показать описание
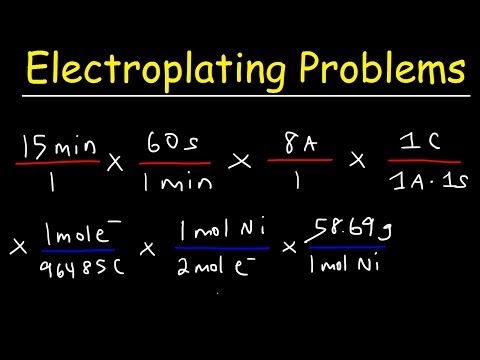
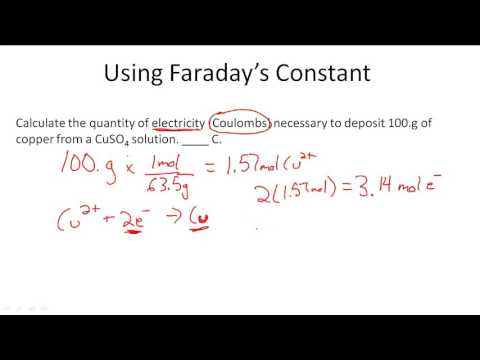
A quantity of 0.300 g of copper was deposited from a CuSO4 solution by passing a current of 3.00 A through the solution for 304 s. Calculate the value of the Faraday constant.
————————————
INTERVIEW
————————————
INTERDISCIPLINARY ART LECTURE:
————————————
————————————
INTERVIEW
————————————
INTERDISCIPLINARY ART LECTURE:
————————————
 0:20:47
0:20:47
 0:07:42
0:07:42
 0:03:20
0:03:20
 0:09:04
0:09:04
 0:08:03
0:08:03
 0:13:01
0:13:01
 0:01:27
0:01:27
 0:09:53
0:09:53
 0:38:36
0:38:36
 0:04:01
0:04:01
 0:20:10
0:20:10
 0:09:11
0:09:11
 0:08:17
0:08:17
 0:03:18
0:03:18
 0:02:57
0:02:57
 0:13:16
0:13:16
 0:10:18
0:10:18
 0:07:27
0:07:27
 0:15:18
0:15:18
 0:14:59
0:14:59
 0:02:53
0:02:53
 0:03:02
0:03:02
 0:06:57
0:06:57
 0:01:59
0:01:59