filmov
tv
A.2 Faraday constant (SL)

Показать описание
Understandings:
The relationship between charge and the number of moles of electrons is given by Faraday’s constant, F.
Applications and skills:
Solving stoichiometric problems using Faraday’s constant based on mass deposits in electrolysis.
The relationship between charge and the number of moles of electrons is given by Faraday’s constant, F.
Applications and skills:
Solving stoichiometric problems using Faraday’s constant based on mass deposits in electrolysis.
A.2 Faraday constant (SL)
The Faraday Constant
Electrochem Eng L01-18 Faraday constant and Faraday's law
The Faraday Constant and the Avogadro constant
Faraday constant
What is Faraday Constant
Calculate Faraday's Constant - Electroplating 002
The Secret of Faraday Constant | Faraday laws | Chemistry Tutor |
Faraday Constant Question | Electrolysis | A Level 9701 | Chemistry
26 July 2021 - A2 Chemistry - Electrochemistry - Faraday Constant - Electrode Potential
Yr12 Determine FARADAY’S Constant
Faraday's constant and mol of electrons
Faraday's constant and Avogadro's number lab
CHEMISTRY: ELECTROLYSIS- Faraday's constant.
Quantitative Electrolysis - Worked example with solutions (Part 2)
what is faraday's constant | faraday's constant electrochemistry | faraday constant calcul...
19.3b Using the Faraday constant
HOW TO USE FARADAY'S CONSTANT in electrolysis
CSEC Chemistry - Electrolysis - Faraday’s Constant - Part 3
Electrolysis calculations, Faraday’s laws
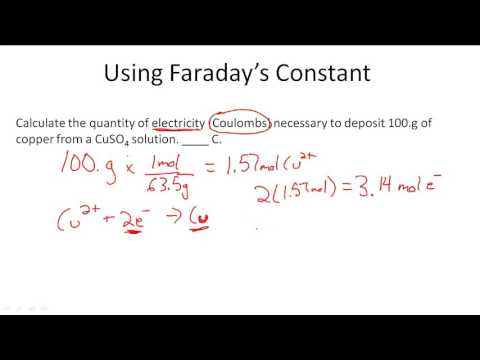
Using Faraday's Constant
Faraday's Constant and Electroplating
Dimensional formula of Faraday constant
determining the Faraday constant by Hemn Afandy
Комментарии
 0:03:20
0:03:20
 0:17:42
0:17:42
 0:06:05
0:06:05
 0:12:47
0:12:47
 0:01:02
0:01:02
 0:00:39
0:00:39
 0:07:42
0:07:42
 0:02:57
0:02:57
 0:03:18
0:03:18
 0:43:44
0:43:44
 0:09:53
0:09:53
 0:04:45
0:04:45
 0:06:09
0:06:09
 0:01:08
0:01:08
 0:07:39
0:07:39
 0:02:41
0:02:41
 0:04:30
0:04:30
 0:15:00
0:15:00
 0:01:01
0:01:01
 0:09:04
0:09:04
 0:02:53
0:02:53
 0:38:36
0:38:36
 0:01:30
0:01:30
 0:08:31
0:08:31