filmov
tv
9.1 Oxidation states (SL)

Показать описание
9.1 Oxidation states
Applications and skills:
Deduction of the oxidation states of an atom in an ion or a compound.
Deduction of the name of a transition metal compound from a given formula, applying oxidation numbers represented by Roman numerals.
Applications and skills:
Deduction of the oxidation states of an atom in an ion or a compound.
Deduction of the name of a transition metal compound from a given formula, applying oxidation numbers represented by Roman numerals.
9.1 Oxidation states (SL)
S3.1.6 How do I Assign Oxidation States? [SL IB Chemistry]
S3.1.9 Oxidation states of the transition elements (HL)
How to Find Oxidation Numbers (Rules and Examples)
S3.1.8/9 Why do Transition Metals Have Variable Oxidation States? [HL IB Chemistry]
Oxidation States IB Chemistry Topic 9.1
S3.1.5 Oxidation states
IB Chemistry Topic 9 Redox processes Topic 9.1 Oxidation and reduction SL
9.1 Definitions of oxidation and reduction (SL)
9.1.1 Oxidation States
9 1 oxidation reduction 100 quality W2014
9.1.2 Assigning Oxidation Numbers IB Chemistry
Using Oxidation Numbers | A Level & SL IB Chemistry
9.1.2 Deduce the oxidation number of an element in a compound.
4.1.5 State that transition elements can form more than one ion IB Chemistry SL
√ Oxidation States | Production of Materials
Discussion 9-1 Intro to Redox Reactions SL/HL
IB Chemistry Topic 9.1: Oxidation & Reduction
Oxidation Numbers
9.1 Oxidizing and reducing agents (SL)
9.1 Introduction to Oxidation and Reduction
13.2.3 The existence of variable oxidation number in ions of transition metals IB Chemistry HL
Oxidation states of carbon | Resonance and acid-base chemistry | Organic chemistry | Khan Academy
Most💯 Important Step Before any Procedure 🔥
Комментарии
 0:07:21
0:07:21
 0:08:43
0:08:43
 0:04:30
0:04:30
 0:07:00
0:07:00
 0:05:04
0:05:04
 0:05:49
0:05:49
 0:07:52
0:07:52
 0:16:45
0:16:45
 0:02:59
0:02:59
 0:07:40
0:07:40
 0:24:20
0:24:20
 0:05:02
0:05:02
 0:13:36
0:13:36
 0:06:24
0:06:24
 0:01:46
0:01:46
 0:26:22
0:26:22
 0:24:13
0:24:13
 0:10:13
0:10:13
 0:02:51
0:02:51
 0:03:16
0:03:16
 0:04:02
0:04:02
 0:03:09
0:03:09
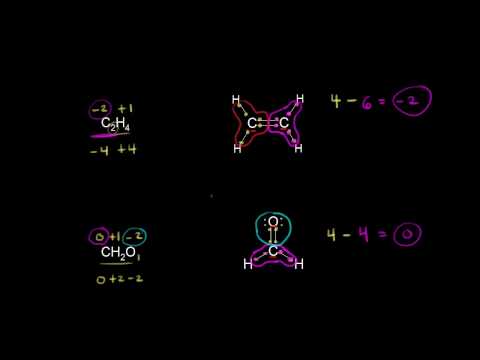 0:11:11
0:11:11
 0:00:16
0:00:16