filmov
tv
1.2/S1.4.1 Determine the number of particles/amount of substance (in moles) [SL IB Chemistry]

Показать описание
Number of particles = moles x 6.022x10^23
How many eggs in half a dozen eggs ?
An egg has a mass of 50g, what is the mass of 2 dozen eggs?
How many dozen is 18 eggs?
If you can answer these questions, you can do those based on moles. The maths is exactly the same, just using bigger numbers!
How many eggs in half a dozen eggs ?
An egg has a mass of 50g, what is the mass of 2 dozen eggs?
How many dozen is 18 eggs?
If you can answer these questions, you can do those based on moles. The maths is exactly the same, just using bigger numbers!
1.2/S1.4.1 Determine the number of particles/amount of substance (in moles) [SL IB Chemistry]
Add 1/2 + 1/4
Sum of natural numbers 1 to 100
His reaction when he sees her FEET for the first time…😳 #Shorts
Arithmetic Progression (AP), find the 1st, 10th and nth term.
Trying transition video for the first time 💙😂 || #transformation #transition #shorts #viralvideo...
1+2+3+...+100 = ?
Numberphile v. Math: the truth about 1+2+3+...=-1/12
N 1/2 of SW 1/4 AND SW 1/4 of NE 1/4 Has How Many Acres? | Real Estate Exam Prep
Indian vs Japanese Maths 🔥| Vedic Maths Trick for Fast Calculation | Speed Maths #trending #shorts...
Carbon Laser Peel treatment at Skinaa Clinic | Viral #shorts
Groups of Permutations
Discrete Random Variables The Expected Value of X and VarX
Dekho Note Counting Machine me kya ho rha hai.
Probability & Statistics (41 of 62) Permutations and Combinations - Example 6
1st yr. Vs Final yr. MBBS student 🔥🤯#shorts #neet
Can you solve this 150 years old puzzle? #shorts
Sneha Pareek(topper)checking Jee mains result 2022//score 300/300
Find the Missing Number of an Equivalent Fraction | Numerator & Denominator | Pre-Algebra | Eat ...
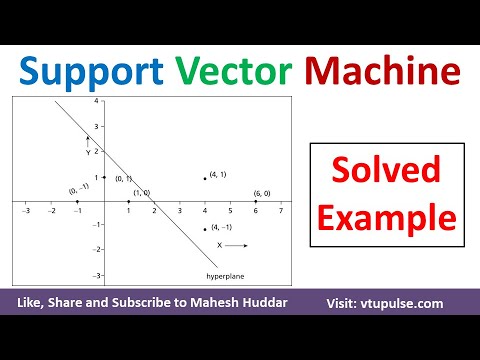
How to draw a hyper plane in Support Vector Machine | Linear SVM – Solved Example by Mahesh Huddar
How to eat Roti #SSB #SSB Preparation #Defence #Army #Best Defence Academy #OLQ
😭A student who didn't qualify jee advanced| subject wise cutoff not clear | #iit #jeemains #jee...
asking minor test marks to allen topper allen kota #allen #allenkota #physicswallah #pw
How to Solve One-Step Equations | One-Step Equation Steps | Math with Mr. J
Комментарии
 0:04:14
0:04:14
 0:01:27
0:01:27
 0:02:26
0:02:26
 0:01:00
0:01:00
 0:07:07
0:07:07
 0:00:15
0:00:15
 0:00:56
0:00:56
 0:41:44
0:41:44
 0:04:14
0:04:14
 0:00:13
0:00:13
 0:00:30
0:00:30
 0:11:07
0:11:07
 0:05:33
0:05:33
 0:00:16
0:00:16
 0:04:54
0:04:54
 0:00:20
0:00:20
 0:00:57
0:00:57
 0:00:35
0:00:35
 0:06:34
0:06:34
 0:08:27
0:08:27
 0:00:16
0:00:16
 0:00:13
0:00:13
 0:00:30
0:00:30
 0:06:54
0:06:54