filmov
tv
Polarity of Water Molecule Explained

Показать описание
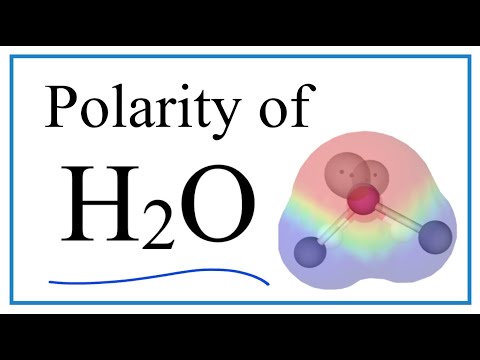
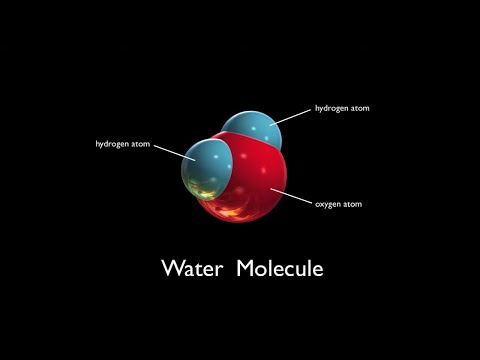
Water is a polar molecule and has a net dipole. If we look at the Lewis structure for water, we can determine that the molecular geometry of the water molecule is bent.
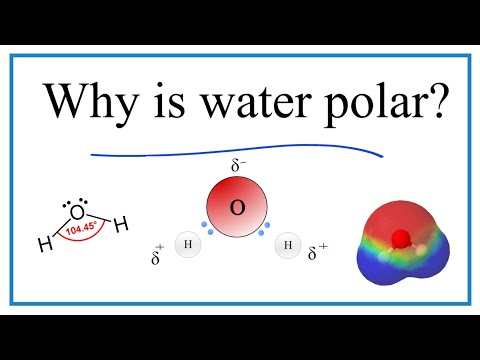
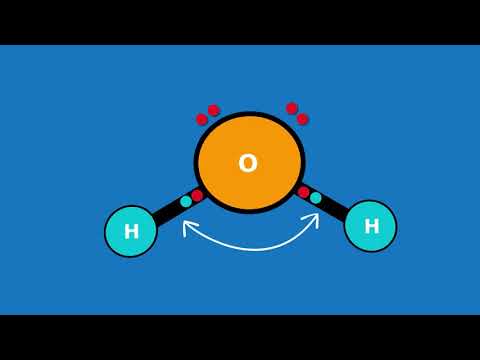
Because H2O has a bent molecular geometry it has a side with the O atom and a side with the H atoms. The electrons in the covalent bond between the O and H atoms aren’t shared equally. They spend more time around the O than with the H atoms. And because electrons are negative, the O becomes a bit more negative. That means the H atoms are more positive. We have poles. So water is a polar molecule.
The polarity of water is responsible for many of it's chemical and physical proprieties.
Looking at the Periodic table for electronegativity values for H and O, we see Oxygen has a value of 3.5. In the case of the water molecule, hydrogen is further away and is 2.1. So the the electrons shared in the bonds between the O atom and the H atoms will spend more time around the O atom.
More Learning Resources
Chapters:
0:00 Intro & Lewis Structure
0:25 Electronegativity Difference and Polarity
1:08 Molecular Geometry Visualized
1:45 Polarity Visualized
Because H2O has a bent molecular geometry it has a side with the O atom and a side with the H atoms. The electrons in the covalent bond between the O and H atoms aren’t shared equally. They spend more time around the O than with the H atoms. And because electrons are negative, the O becomes a bit more negative. That means the H atoms are more positive. We have poles. So water is a polar molecule.
The polarity of water is responsible for many of it's chemical and physical proprieties.
Looking at the Periodic table for electronegativity values for H and O, we see Oxygen has a value of 3.5. In the case of the water molecule, hydrogen is further away and is 2.1. So the the electrons shared in the bonds between the O atom and the H atoms will spend more time around the O atom.
More Learning Resources
Chapters:
0:00 Intro & Lewis Structure
0:25 Electronegativity Difference and Polarity
1:08 Molecular Geometry Visualized
1:45 Polarity Visualized
Комментарии
 0:02:23
0:02:23
 0:03:52
0:03:52
 0:04:25
0:04:25
 0:10:46
0:10:46
 0:00:58
0:00:58
 0:03:03
0:03:03
 0:06:51
0:06:51
 0:05:28
0:05:28
 0:10:53
0:10:53
 0:02:45
0:02:45
 0:02:13
0:02:13
 0:02:37
0:02:37
 0:02:35
0:02:35
 0:03:33
0:03:33
 0:03:47
0:03:47
 0:01:23
0:01:23
 0:00:46
0:00:46
 0:00:37
0:00:37
 0:11:06
0:11:06
 0:04:47
0:04:47
 0:00:15
0:00:15
 0:03:48
0:03:48
 0:01:53
0:01:53
 0:02:25
0:02:25