filmov
tv
Why is water (H2O) a polar molecule?
Показать описание
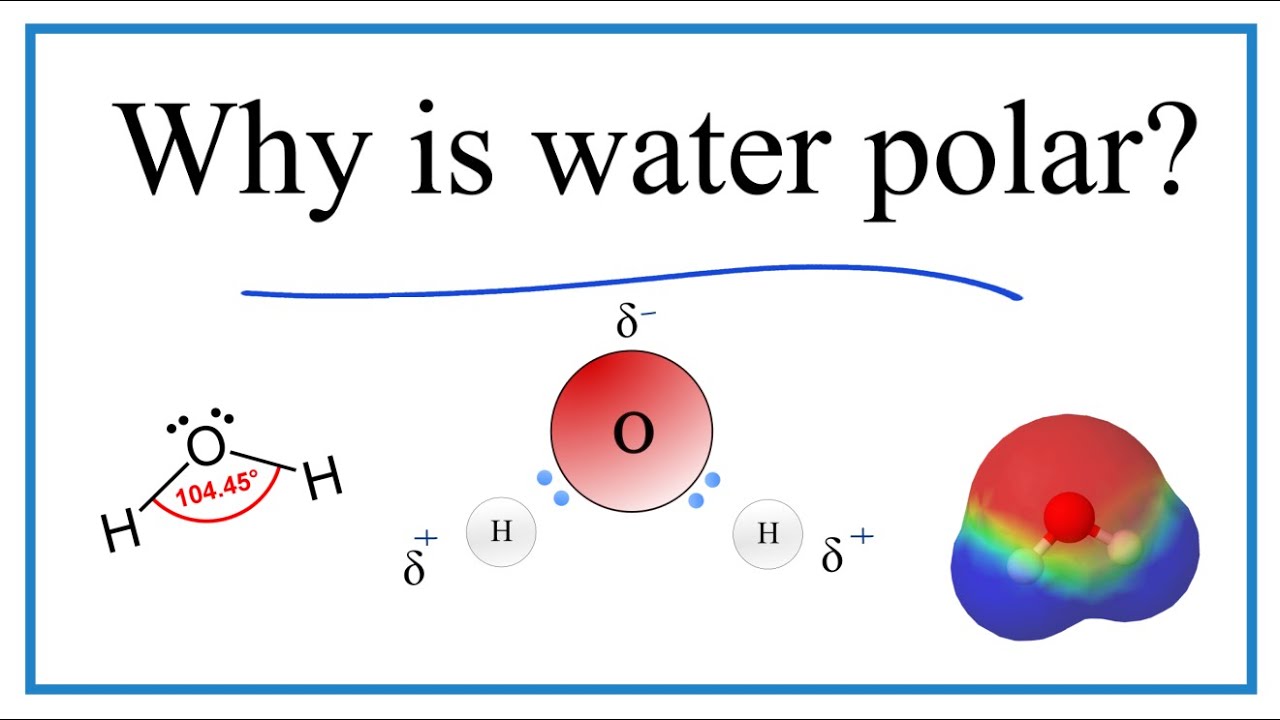
To understand why water is a polar molecule, we need to understand a few key concepts about polarity. First, it we look at the Lewis structure for water. this helps understand the molecular geometry of the water molecule which is bent.
Because H2O has a bent molecular geometry it has a side with the O atom and a side with the H atoms. The electrons in the covalent bond between the O and H atoms aren’t shared equally. They spend more time around the O than with the H atoms. And because electrons are negative, the O becomes a bit more negative. That means the H atoms are more positive. We have poles. So water is a polar molecule.
We can check the EN values on this Periodic Table. Oxygen has a value of 3.5. Hydrogen is further away and is 2.1. So the the electrons in the bond between the O atom and the H atoms will spend more time around the O atom.
More Learning Resources
Because H2O has a bent molecular geometry it has a side with the O atom and a side with the H atoms. The electrons in the covalent bond between the O and H atoms aren’t shared equally. They spend more time around the O than with the H atoms. And because electrons are negative, the O becomes a bit more negative. That means the H atoms are more positive. We have poles. So water is a polar molecule.
We can check the EN values on this Periodic Table. Oxygen has a value of 3.5. Hydrogen is further away and is 2.1. So the the electrons in the bond between the O atom and the H atoms will spend more time around the O atom.
More Learning Resources
Why is water (H2O) a polar molecule?
Facts About Water H2O - Science With Kids
Properties of Water
Why Water is H2O: The Science Explained!
H2O - Water's Molecular Structure - Why is H2O a Polar Molecule? #shorts #h2o #covalentbond
Formation of water Molecule | Water H2O Formation #water #molecule #h2o #covalentbond #short
Every full moon episode of H2O just add water #shorts #h2ojustaddwater #h2o #mermaid #aussie
H2O Explained with Animation in 2024 #viralvideo #trending
The Incredible Water Molecule [H2O Structure and Properties]
Water is not H2O
The H2O Dipole
H2O just add water core 💀🧜🏼♀️ #shorts #h2o #h2ojustaddwater #core #mermaid
H2O Behind the scenes: Wardrobe and Makeup #h2ojustaddwater #mermaids #movie
The difference one atom of Oxygen makes- H2O & H2O2.
Phoebe Tonkin Remembers H2O Just Add Water | W Magazine
My small water bottle 🫣 #water #drink #h2o #seanhasjokes #tallvsshort
Music H2O Just Add Water characters would listen to
Mermaid VS Pirate: do you know how this ended? #h2ojustaddwater #h2o #mermaids
Will discovers Bella's secret | H2O - Just add Water
Why would HE DO THIS? #h2ojustaddwater #h2o
Is This Rikki's BEST One-Liner??!! #h2o #h2ojustaddwater
NAUR CLEO H2O just add water! #shorts #h2o #h2ojustaddwater #mermaid #aussie #australian
Discovering the water powers | H2O - Just Add Water | #Shorts
Lewis dot structure of water (H2O).
Комментарии
 0:04:25
0:04:25
 0:04:44
0:04:44
 0:06:51
0:06:51
 0:02:45
0:02:45
 0:02:01
0:02:01
 0:00:46
0:00:46
 0:00:39
0:00:39
 0:00:13
0:00:13
 0:04:43
0:04:43
 0:27:34
0:27:34
 0:03:47
0:03:47
 0:00:12
0:00:12
 0:00:30
0:00:30
 0:00:28
0:00:28
 0:00:35
0:00:35
 0:00:11
0:00:11
 0:00:20
0:00:20
 0:00:14
0:00:14
 0:00:36
0:00:36
 0:00:26
0:00:26
 0:00:24
0:00:24
 0:00:30
0:00:30
 0:00:17
0:00:17
 0:00:24
0:00:24