filmov
tv
Calculate the number of photons in \( 6.63 \mathrm{~J} \) of radiation energy of frequency \( 10...

Показать описание
Calculate the number of photons in \( 6.63 \mathrm{~J} \) of radiation energy of frequency \( 10^{12} \mathrm{~Hz} \), Given \( h=6.63 \times 10^{-34} \mathrm{~J} \mathrm{~s} \).
 0:09:39
0:09:39
 0:11:53
0:11:53
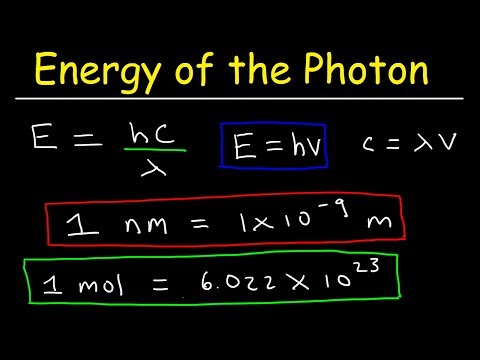 0:11:06
0:11:06
 0:05:15
0:05:15
 0:02:06
0:02:06
 0:04:27
0:04:27
 0:02:36
0:02:36
 0:02:09
0:02:09
 0:02:03
0:02:03
 0:03:08
0:03:08
 0:07:42
0:07:42
 0:07:01
0:07:01
 0:02:59
0:02:59
 0:08:05
0:08:05
 0:07:26
0:07:26
 0:05:01
0:05:01
 0:03:37
0:03:37
 0:00:57
0:00:57
 0:01:52
0:01:52
 0:02:35
0:02:35
 0:03:13
0:03:13
 0:36:02
0:36:02
 0:02:02
0:02:02
 0:01:52
0:01:52