filmov
tv
How to Find the Wavelength, Frequency, Energy and Photons | Study Chemistry With Us

Показать описание
I'll teach you how to calculate the wavelength, frequency, energy, number of photons, energy of mole of photons and more. We'll go over how to properly use each formula used in the electromagnetic spectrum and of course, everything is step by step.
📝 DOWNLOAD THE STUDY PLAN
📗 FREE CHEMISTRY SURVIVAL GUIDE
📘 FREE ORGANIC CHEMISTRY SURVIVAL GUIDE
💯 HERE'S HOW TO PASS ORGANIC CHEMISTRY 🎉
👉 MORE CHEMISTRY RESOURCES I CREATED 👈
🎓 CHECKOUT MY COMPLETE CHEMISTRY GUIDES:
📕 Thermochemistry Guide
📗 Acids and Bases Guide
📘 Naming Compounds and Acids Guide
📙 Dimensional Analysis, Significant Figures, and Density Guide
📕 Gas Laws Guide
📗 Stoichiometry Guide
📘 Redox Reactions Guide
📙 Molarity Guide
📕 Limiting Reactants Guide
📗 Lewis Structures Guide
📘 Kinetics Guide
📙 Titrations Guide
📕 Matter, Atomic Structure, Empirical and Molecular Formulas Guide
🙌 This was my go-to homework help when I was in school. Chegg Study is one of my favorites.
📚 I made the mistake of buying all of my textbooks, I wish I had the option of renting them. Thankfully you do, with Chegg Textbook Rentals.
💁♀️ HI I'M MELISSA MARIBEL
I help students pass Chemistry and Organic Chemistry. I used to struggle with this subject, so when I finally graduated with a bachelor's degree in Chemistry, I became a tutor so that you wouldn't have to struggle like I did. I know that with the right help, YOU CAN LEARN ANYTHING!
DISCLAIMER: Some links in the description are affiliate links, which means that if you buy from those links, I’ll receive a small commission. This helps support the channel and allows me to continue making videos like this. Thanks for the support!
Questions Covered in this Video:
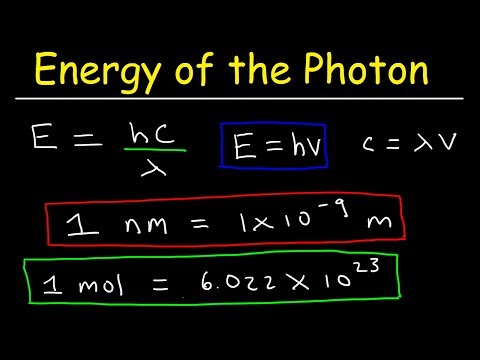
1) Calculate the energy of X-rays with a wavelength of 15.0 nm.
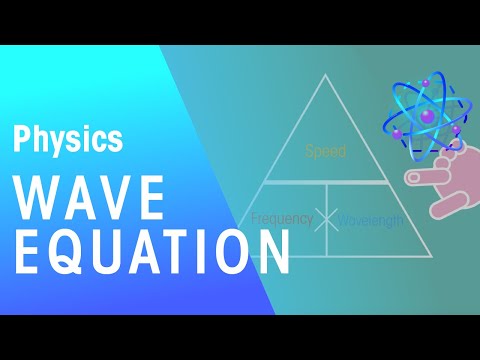
2) What is the wavelength in meters of an FM radio wave with a frequency of 102.5 MHz?
3) If the wavelength of a photon is 552 nm. What is the energy of one mole of photons?
4) How many photons are contained in a flash of green light (525 nm) that contains 189 kJ of energy?
📝 DOWNLOAD THE STUDY PLAN
📗 FREE CHEMISTRY SURVIVAL GUIDE
📘 FREE ORGANIC CHEMISTRY SURVIVAL GUIDE
💯 HERE'S HOW TO PASS ORGANIC CHEMISTRY 🎉
👉 MORE CHEMISTRY RESOURCES I CREATED 👈
🎓 CHECKOUT MY COMPLETE CHEMISTRY GUIDES:
📕 Thermochemistry Guide
📗 Acids and Bases Guide
📘 Naming Compounds and Acids Guide
📙 Dimensional Analysis, Significant Figures, and Density Guide
📕 Gas Laws Guide
📗 Stoichiometry Guide
📘 Redox Reactions Guide
📙 Molarity Guide
📕 Limiting Reactants Guide
📗 Lewis Structures Guide
📘 Kinetics Guide
📙 Titrations Guide
📕 Matter, Atomic Structure, Empirical and Molecular Formulas Guide
🙌 This was my go-to homework help when I was in school. Chegg Study is one of my favorites.
📚 I made the mistake of buying all of my textbooks, I wish I had the option of renting them. Thankfully you do, with Chegg Textbook Rentals.
💁♀️ HI I'M MELISSA MARIBEL
I help students pass Chemistry and Organic Chemistry. I used to struggle with this subject, so when I finally graduated with a bachelor's degree in Chemistry, I became a tutor so that you wouldn't have to struggle like I did. I know that with the right help, YOU CAN LEARN ANYTHING!
DISCLAIMER: Some links in the description are affiliate links, which means that if you buy from those links, I’ll receive a small commission. This helps support the channel and allows me to continue making videos like this. Thanks for the support!
Questions Covered in this Video:
1) Calculate the energy of X-rays with a wavelength of 15.0 nm.
2) What is the wavelength in meters of an FM radio wave with a frequency of 102.5 MHz?
3) If the wavelength of a photon is 552 nm. What is the energy of one mole of photons?
4) How many photons are contained in a flash of green light (525 nm) that contains 189 kJ of energy?
Комментарии
 0:04:05
0:04:05
 0:11:36
0:11:36
 0:03:13
0:03:13
 0:02:09
0:02:09
 0:05:53
0:05:53
 0:12:43
0:12:43
 0:36:02
0:36:02
 0:02:35
0:02:35
 0:47:00
0:47:00
 0:08:32
0:08:32
 0:04:52
0:04:52
 0:11:06
0:11:06
 0:01:41
0:01:41
 0:08:20
0:08:20
 0:00:36
0:00:36
 0:03:35
0:03:35
 0:03:24
0:03:24
 0:13:35
0:13:35
 0:01:45
0:01:45
 0:04:59
0:04:59
 0:04:36
0:04:36
 0:11:21
0:11:21
 0:04:43
0:04:43
 0:06:29
0:06:29