filmov
tv
Equilibrium (A-level IB Chemistry)

Показать описание
Outlining what is meant by dynamic equilibrium, the features of a system at equilibrium and look at what ‘positions of equilibrium’ are. The terms heterogeneous and homogenous are used to describe different types of system. Example of hydrogen, iodine and hydrogen iodide system is shown and explained in terms of rates of reaction.
Recap: 00:31
Reversible Reactions: 01:01
Example - Hydrogen and Iodine: 02:41
Dynamic Equilibrium: 04:45
Features of a System at Equilibrium: 05:25
Positions of Equilibrium: 07:34
Heterogeneous and Homogeneous: 09:12
Summary: 10:30
Thank you for watching - if you found the video useful, please like and subscribe!
Recap: 00:31
Reversible Reactions: 01:01
Example - Hydrogen and Iodine: 02:41
Dynamic Equilibrium: 04:45
Features of a System at Equilibrium: 05:25
Positions of Equilibrium: 07:34
Heterogeneous and Homogeneous: 09:12
Summary: 10:30
Thank you for watching - if you found the video useful, please like and subscribe!
 0:13:12
0:13:12
 0:10:47
0:10:47
 0:06:48
0:06:48
 0:12:53
0:12:53
 0:13:26
0:13:26
 0:05:11
0:05:11
 0:17:24
0:17:24
 0:14:30
0:14:30
 0:13:09
0:13:09
 0:12:36
0:12:36
 0:06:01
0:06:01
 0:15:37
0:15:37
 0:04:19
0:04:19
 0:05:04
0:05:04
 0:11:37
0:11:37
 0:07:00
0:07:00
 0:08:17
0:08:17
 0:21:13
0:21:13
 0:29:21
0:29:21
 0:10:49
0:10:49
 0:38:53
0:38:53
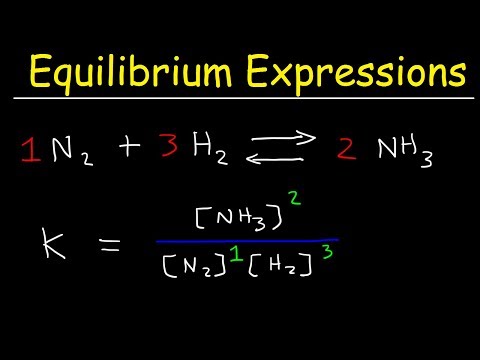 0:05:24
0:05:24
 0:10:13
0:10:13
 0:04:51
0:04:51