filmov
tv
Intermolecular Forces for H2O (Water)

Показать описание
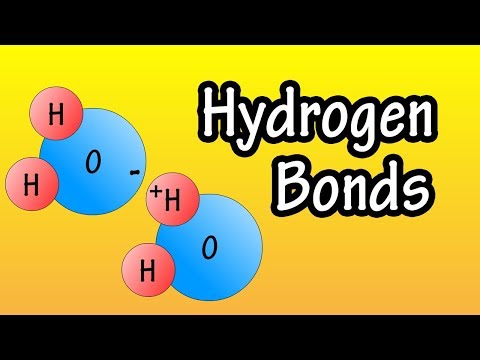
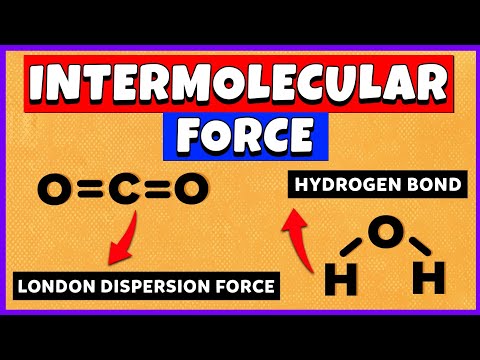
In this video we’ll identify the intermolecular forces for H2O (water). Using a flowchart to guide us, we find that H2O is a polar molecule. It also has the Hydrogen atoms bonded to an Oxygen atom. Therefore H2O the main intermolecular force is Hydrogen Bonding (note that H2O also has Dipole-Dipole and London Dispersion Forces).
In determining the intermolecular forces present for H2O we follow these steps:
- Determine if there are ions present. Since H2O is a molecule and there is no + or – sign after the H2O we can say that it is not an ion.
Useful Resources:
In determining the intermolecular forces present for H2O we follow these steps:
- Determine if there are ions present. Since H2O is a molecule and there is no + or – sign after the H2O we can say that it is not an ion.
Useful Resources:
Intermolecular Forces for H2O (Water)
Hydrogen Bonds In Water Explained - Intermolecular Forces
Intermolecular Forces and Boiling Points
Intermolecular Forces of Water
The H2O Dipole
Water streams tend to join because of cohesion | Molecular Force | Chemistry
Why H₂O is Liquid and H₂S is Gas The Science of Molecular Forces Explained!
What type of intermolecular force would water molecules have?
Hydrogen Bonding in Water
Hydrogen Bonds - What Are Hydrogen Bonds - How Do Hydrogen Bonds Form
Intermolecular Forces and Water
General Chemistry 2: Properties of Liquid and Intermolecular Forces
12 1 Hydrogen bonding in water
How to identify intermolecular forces?
Water and ice | Intermolecular Forces | meriSTEM
Lecture 2: Water and Intermolecular Forces - 08/25/20
Difference in Boiling Points for H2O and H2S
Hydrogen Bonding in Water
What intermolecular forces are present in water?
Properties of Water
Why is the Boiling Point of water (H2O) so high?
Intermolecular Forces
Intermolecular forces lab - water
Hydrogen bonds in Water Molecules
Комментарии
 0:02:01
0:02:01
 0:10:54
0:10:54
 0:10:54
0:10:54
 0:06:45
0:06:45
 0:03:47
0:03:47
 0:01:37
0:01:37
 0:03:45
0:03:45
 0:09:17
0:09:17
 0:06:53
0:06:53
 0:02:48
0:02:48
 0:04:49
0:04:49
 0:04:47
0:04:47
 0:02:49
0:02:49
 0:08:05
0:08:05
 0:04:13
0:04:13
 0:57:40
0:57:40
 0:03:25
0:03:25
 0:03:28
0:03:28
 0:01:47
0:01:47
 0:06:51
0:06:51
 0:05:18
0:05:18
 0:29:50
0:29:50
 0:00:38
0:00:38
 0:01:06
0:01:06