filmov
tv
IB Chemistry Atomic Structure Revision Workshop HL/SL (Topic 2/12)

Показать описание
In this video I go through practice questions on the main subtopics for Atomic structure step-by-step so you can work alongside me :) (You'll find all the worksheets I use linked below)
00:00 Introduction
01:04 SL Equations
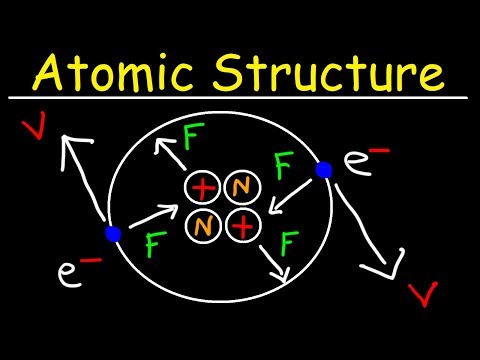
03:13 Subatomic particles
05:45 Calculating Ar
09:58 Electron Configuration
19:37 Hydrogen Emission Spectrum
23:12 HL Equations
27:57 First Ionisation Energy
39:42 Calculating First Ionisation Energy
46:24 Outro
00:00 Introduction
01:04 SL Equations
03:13 Subatomic particles
05:45 Calculating Ar
09:58 Electron Configuration
19:37 Hydrogen Emission Spectrum
23:12 HL Equations
27:57 First Ionisation Energy
39:42 Calculating First Ionisation Energy
46:24 Outro
IB Chemistry Atomic Structure Revision Workshop HL/SL (Topic 2/12)
Electron Configuration [IB Chemistry SL/HL]
The Nuclear Atom [IB Chemistry SL/HL]
IB Chem Topic 2 Revision: Atomic Structure
IB Chem HL Topic 12 Revision: Atomic Structure
GENERAL CHEMISTRY explained in 19 Minutes
Covalent Bonding [IB Chemistry SL/HL]
Topic 3 Periodicity: Everything You Need to Know for the IB Exam
Chemistry - Atomic Structure - EXPLAINED!
IB Chemistry SL Topic 2: Revision Lecture
The Periodic Table - Classification of Elements [IB Chemistry SL/HL]
VSEPR & Molecular Polarity [IB Chemistry SL/HL]
Ionic Bonding [IB Chemistry SL/HL]
IB Chemistry Topic 2 Atomic structure 2.1 The nuclear atom
IB Chemistry: Atomic Structure Overview
A Level Chemistry is EFFORTLESS Once You Learn This
The Atom - Course Revision(IB Chemistry) Fundamentals
Tips and Tricks to solving IB Chemistry Paper 1 questions (sample 1)
The Mole - [IB Chemistry SL/HL]
Metallic Bonding [IB Chemistry SL/HL]
How to get a 7 in IB Chemistry in 2024
IB Chemistry SL HL Topic 2 Atomic structure full PPT (OLD VERSION)
Intermolecular Forces [IB Chemistry SL/HL]
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Комментарии
 0:46:50
0:46:50
 0:11:05
0:11:05
 0:13:29
0:13:29
 0:22:38
0:22:38
 0:05:11
0:05:11
 0:18:49
0:18:49
 0:14:30
0:14:30
 0:20:14
0:20:14
 0:11:45
0:11:45
 0:33:42
0:33:42
 0:14:46
0:14:46
 0:12:48
0:12:48
 0:14:09
0:14:09
 0:08:14
0:08:14
 0:14:32
0:14:32
 0:05:30
0:05:30
 0:04:46
0:04:46
 0:19:44
0:19:44
 0:12:38
0:12:38
 0:05:25
0:05:25
 0:09:58
0:09:58
 0:25:36
0:25:36
 0:11:03
0:11:03
 0:08:42
0:08:42