filmov
tv
Quantum Chemistry 1.5 - Bohr Hydrogen Model 2: Energy

Показать описание
Short lecture on the Bohr hydrogen model energy.
Bohr assumed the electron in the hydrogen atom travels in a fixed circular orbit with quantized angular momentum. By balancing the Coulomb force and centrifugal force acting on the electron, we can derive the radii, velocities, and energies of its allowed orbits. Transitions between these allowed energy levels leads to an expression which is in nearly exact agreement with the Rydberg formula, giving a value of the Rydberg constant which has extremely low error (~0.001%).
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
Bohr assumed the electron in the hydrogen atom travels in a fixed circular orbit with quantized angular momentum. By balancing the Coulomb force and centrifugal force acting on the electron, we can derive the radii, velocities, and energies of its allowed orbits. Transitions between these allowed energy levels leads to an expression which is in nearly exact agreement with the Rydberg formula, giving a value of the Rydberg constant which has extremely low error (~0.001%).
--- About TMP Chem ---
All TMP Chem content is free for everyone, everywhere, and created independently by Trent Parker.
--- Video Links ---
--- Social Links ---
--- Equipment ---
Microphone: Blue Yeti USB Microphone
Drawing Tablet: Wacom Intuos Pen and Touch Small
Drawing Program: Autodesk Sketchbook Express
Screen Capture: Corel Visual Studio Pro X8
Bohr Model of the Hydrogen Atom, Electron Transitions, Atomic Energy Levels, Lyman & Balmer Seri...
Bohr Model of the Hydrogen Atom
Quantum Chemistry 1.5 - Bohr Hydrogen Model 2: Energy
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Brian Cox explains quantum mechanics in 60 seconds - BBC News
Quantum Chemistry 1.4 - Bohr Hydrogen Model 1: Radius (Old Version)
What Are The Different Atomic Models? Dalton, Rutherford, Bohr and Heisenberg Models Explained
The Schrödinger Equation Explained in 60 Seconds
Applied Chemistry-A, Atomic Structure and Chemical Bonding and Solutions: Chapter-1 [ Lec-11]
How small are atoms?
A Better Way To Picture Atoms
Bohr's Atomic Model
Shells, Subshells, and Orbitals - BIOLOGY/CHEMISTRY EP5
Quantum Chemistry 1.4 - Bohr Hydrogen Model 1: Radius
Bohr's Atomic Model | Chemistry
Quantum Numbers | What are the 4 Quantum Numbers? Chemistry
The History of Atomic Chemistry: Crash Course Chemistry #37
Planck's Quantum Theory | Chemistry
Quantum Numbers
Isaac Newton's INSANE Sleep Habits 😬
Bohr's atomic model |⚡3d animation | Class 9, Chemistry |
Gen Chem 1 Module 5 Part 5 Bohr Model fo the Atom
What is the Bohr model of the atom?
5. Shell Models and Quantum Numbers (Intro to Solid-State Chemistry)
Комментарии
 0:21:44
0:21:44
 0:04:50
0:04:50
 0:11:09
0:11:09
 0:08:42
0:08:42
 0:01:22
0:01:22
 0:14:59
0:14:59
 0:07:04
0:07:04
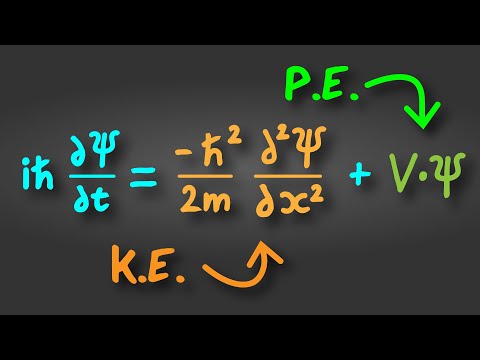 0:01:00
0:01:00
 0:55:19
0:55:19
 0:00:48
0:00:48
 0:05:35
0:05:35
 1:12:19
1:12:19
 0:09:23
0:09:23
 0:08:57
0:08:57
 0:21:11
0:21:11
 0:12:10
0:12:10
 0:09:42
0:09:42
 0:10:24
0:10:24
 0:12:16
0:12:16
 0:00:24
0:00:24
 0:04:38
0:04:38
 0:17:15
0:17:15
 0:27:12
0:27:12
 0:47:54
0:47:54