filmov
tv
How to calculate ppm | ppm calculation
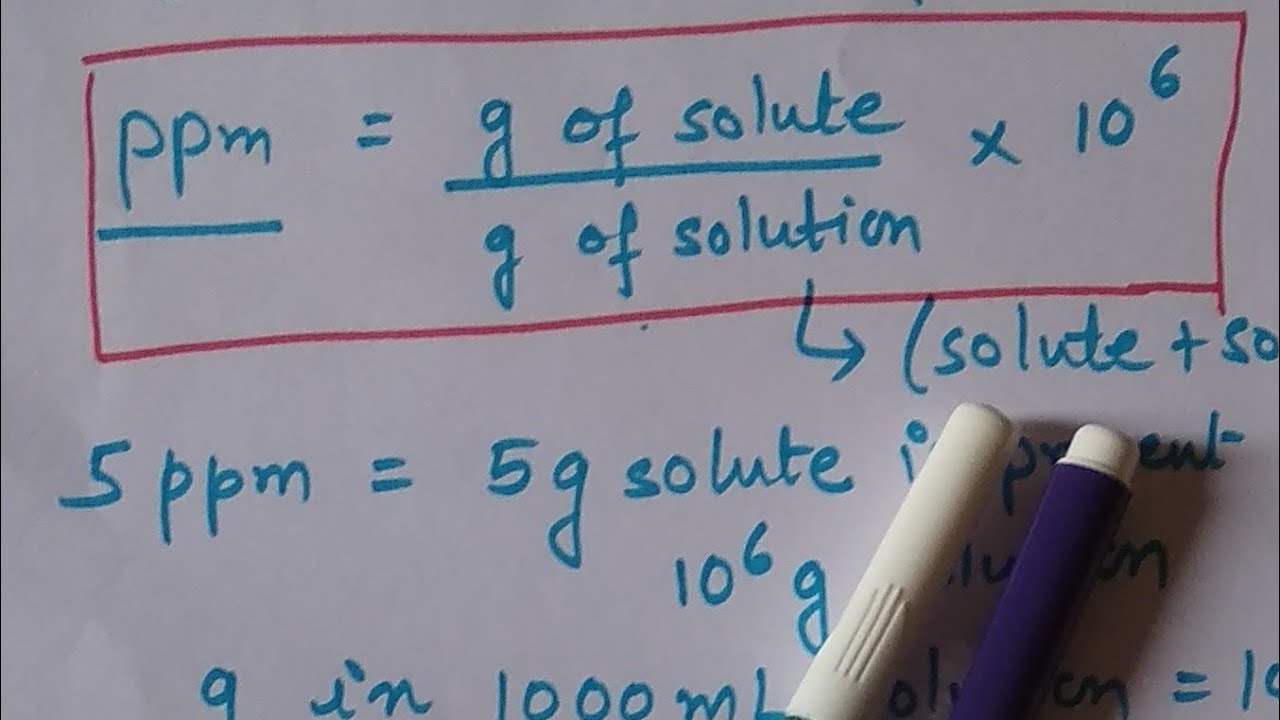
Показать описание
Hello everyone,
Parts per million(ppm) is a concentration term that we use for very dilute solutio
n. So understanding the concept of ppm with its formula used for calculation is discussed here. Also, you will get to learn, how to prepare a given solution in terms of parts per million.
Keep watching.
Thank you.
Happy Learning.
Parts per million(ppm) is a concentration term that we use for very dilute solutio
n. So understanding the concept of ppm with its formula used for calculation is discussed here. Also, you will get to learn, how to prepare a given solution in terms of parts per million.
Keep watching.
Thank you.
Happy Learning.
How to calculate ppm | ppm calculation
Parts Per Million (ppm) and Parts Per Billion (ppb) - Solution Concentration
Calculate ppm for a solution (parts per million) using formula
How to calculate concentration in PPM
Introduction to Calculating the Parts per Million (ppm) Concentration
How to prepare 1ppm, 10ppm, 100ppm and 1000ppm solution | ppm solution preparation
How To Calculate PPM?
How To Convert PPM to Molarity
Learning session: Extending Structure PPM with Timesheets and Capacity Planner
How to calculate ppm?
What are pH, EC, TDS, and PPM and How Are They Connected?
Parts Per Million (PPM) ? Alakh Pandey Sir | @AlakhSirHighlights
1 ppm solution | one ppm solution preparatio | how to prepare 1 ppm solution
How to Measure Parts Per Million in Chemistry : Solving Math Problems
PPM solution | How to prepare 10 ppm solution,20ppm,30ppm, 40ppm,50ppm solutions | ppm calculation
What is PPM || How to calculate PPM || PPM explanation in tamil || Japanese industrial concepts ||
100 ppm solution | 100 ppm solution preparation | make 100 ppm solution
What is PPM | PPM Definition | How to Calculate PPM | PPM Calculation Sheet & Formula | AYT Indi...
Module 2 - 8.1 - Concentration ppm to grams per litre
How to prepare 50 ppm, 100 ppm, 200 ppm solution | PPM Solution | 1000 ppm solution |10 ppm solution
conversion from % to ppm
10 ppm solution | how to prepare 10 ppm solution | how to make 10 ppm solution | make ppm solution
PERCENT CONCENTRATION (PPM & PPB)
What is PPM?| Parts Per Million Calculation | Quality Rejection| NC Product | Explained with example
Комментарии
 0:21:21
0:21:21
 0:11:00
0:11:00
 0:05:29
0:05:29
 0:06:18
0:06:18
 0:04:54
0:04:54
 0:03:18
0:03:18
 0:00:32
0:00:32
 0:11:03
0:11:03
 0:43:02
0:43:02
 0:00:37
0:00:37
 0:22:08
0:22:08
 0:01:21
0:01:21
 0:03:19
0:03:19
 0:01:58
0:01:58
 0:08:02
0:08:02
 0:05:35
0:05:35
 0:02:59
0:02:59
 0:10:36
0:10:36
 0:03:55
0:03:55
 0:05:56
0:05:56
 0:01:19
0:01:19
 0:03:06
0:03:06
 0:02:00
0:02:00
 0:01:49
0:01:49