filmov
tv
How To Convert PPM to Molarity

Показать описание
This chemistry video tutorial explains how to convert the solution concentration from parts per million or ppm to Molarity.
Heating Curve Chemistry Problems:
Final Temperature - Ice Water Mixture:
Molarity, Molality, Density, & Mass %:
Normality & Equivalent Weight:
_________________________________
PPM and PPB Concentrations:
How To Convert PPM to Molarity:
Enthalpy of Solution & Hydration:
Solubility Vs Concentration:
Solubility Curves:
___________________________________
Henry's Law & Gas Solubility:
Vapor Pressure & Clausius Equation:
Raoult's Law - Vapor Pressure:
Colligative Properties:
Chemical Kinetics Initial Rate Method:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Heating Curve Chemistry Problems:
Final Temperature - Ice Water Mixture:
Molarity, Molality, Density, & Mass %:
Normality & Equivalent Weight:
_________________________________
PPM and PPB Concentrations:
How To Convert PPM to Molarity:
Enthalpy of Solution & Hydration:
Solubility Vs Concentration:
Solubility Curves:
___________________________________
Henry's Law & Gas Solubility:
Vapor Pressure & Clausius Equation:
Raoult's Law - Vapor Pressure:
Colligative Properties:
Chemical Kinetics Initial Rate Method:
_________________________________
Final Exams and Video Playlists:
Full-Length Videos and Worksheets:
Chemistry PDF Worksheets:
Комментарии
 0:11:03
0:11:03
 0:11:00
0:11:00
 0:03:55
0:03:55
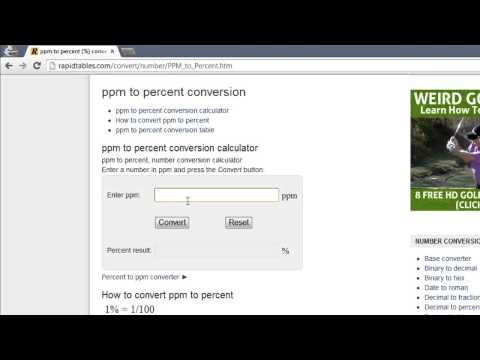 0:00:56
0:00:56
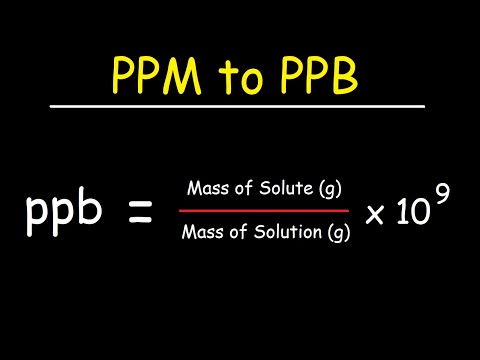 0:05:02
0:05:02
 0:00:30
0:00:30
 0:21:21
0:21:21
 0:01:56
0:01:56
 0:01:31
0:01:31
 0:10:29
0:10:29
 0:01:19
0:01:19
 0:02:15
0:02:15
 0:02:03
0:02:03
 0:06:26
0:06:26
 0:01:14
0:01:14
 0:03:38
0:03:38
 0:00:52
0:00:52
 0:05:39
0:05:39
 0:05:29
0:05:29
 0:00:46
0:00:46
 0:04:46
0:04:46
 0:01:21
0:01:21
 0:01:16
0:01:16
 0:04:54
0:04:54