filmov
tv
Nomenclature 3 - Naming Alcohols

Показать описание
description
Nomenclature 3 - Naming Alcohols
Naming Alcohols - IUPAC Nomenclature
Naming Alcohols Using IUPAC Rules for Nomenclature
Naming Alcohols
IUPAC Nomenclature of Haloalkanes and Alcohols
19: Naming alcohols
How to Name Alcohols - GCSE chemistry organic
S3.2.5 Naming alcohols
DAY 42 | CHEMISTRY | II PUC | ALCOHOLS, PHENOLS AND ETHERS | L8
9.3 Alcohol Nomenclature
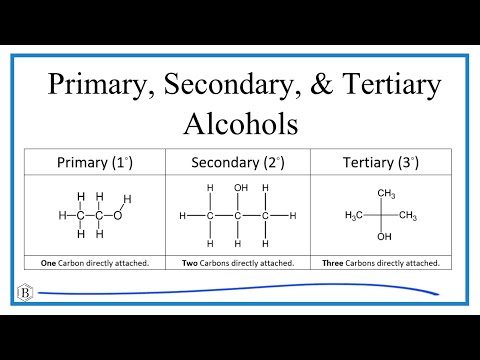
Primary, Secondary, and Tertiary Alcohols: Classification, Examples, & Practice
IUPAC naming of alcohols
12.1 Naming Alcohols | Organic Chemistry
IUPAC Nomenclature of Alkanes - Naming Organic Compounds
Naming Alcohols: IUPAC Nomenclature | Chemistry Tutorials
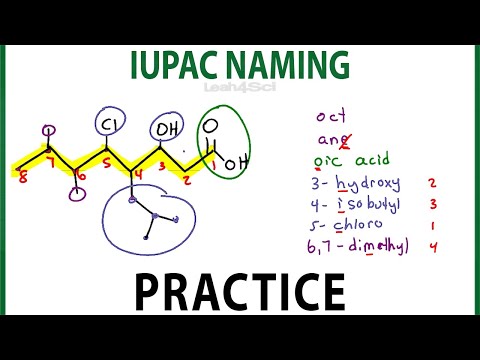
IUPAC Naming Practice - Nomenclature for alkanes, dienes, alcohols and more
Naming alcohols
Examples of naming alcohols and amines
Nomenclature - How to Name Alcohols
Nomenclature of Alcohols
Common Naming of Alcohols
Naming Alcohols Using IUPAC Systematic Nomenclature - Revision for A-Level Chemistry
Naming Alcohols
naming alcohols
Комментарии
 0:09:01
0:09:01
 0:10:42
0:10:42
 0:07:07
0:07:07
 0:07:53
0:07:53
 0:06:12
0:06:12
 0:04:29
0:04:29
 0:03:45
0:03:45
 0:02:53
0:02:53
 0:32:54
0:32:54
 0:06:44
0:06:44
 0:03:45
0:03:45
 0:07:22
0:07:22
 0:08:36
0:08:36
 0:11:18
0:11:18
 0:07:24
0:07:24
 0:10:54
0:10:54
 0:09:02
0:09:02
 0:12:14
0:12:14
 0:12:44
0:12:44
 0:08:30
0:08:30
 0:03:06
0:03:06
 0:05:01
0:05:01
 0:01:42
0:01:42
 0:05:26
0:05:26