filmov
tv
Organic Chemistry: Alcohol Mechanisms & Reactions

Показать описание
Ninja Nerds,
Join us during this lecture where we will be doing practice problems on alcohols, ethers & epoxides!
***PLEASE SUPPORT US***
***EVERY DOLLAR HELPS US GROW & IMPROVE OUR QUALITY***
✎ For general inquiries email us at:
Join us during this lecture where we will be doing practice problems on alcohols, ethers & epoxides!
***PLEASE SUPPORT US***
***EVERY DOLLAR HELPS US GROW & IMPROVE OUR QUALITY***
✎ For general inquiries email us at:
Alcohol Reactions - HBr, PBr3, SOCl2
Organic Chemistry: Alcohol Mechanisms & Reactions
GCSE Chemistry - Alcohols
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24
Introduction to Alcohol Properties and Reactions
12.3 Synthesis of Alcohols | Organic Chemistry
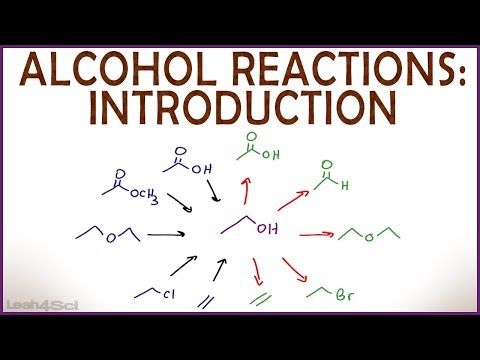
Alcohol Reactions Introduction (Live Recording) Organic Chemistry Review & Practice Session
Organic Chemistry | Alcohol & Ether Mechanisms & Reactions
8 important topics of Organic chemistry | organic chemistry
Alcohols | Alcohols, ethers, epoxides, sulfides | Organic chemistry | Khan Academy
12.6 Substitution Reactions of Alcohols | Organic Chemistry
Naming Alcohols - IUPAC Nomenclature
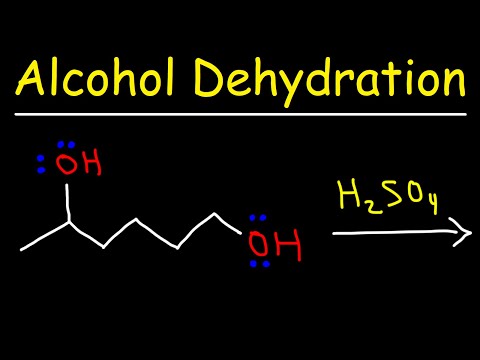
Alcohol Dehydration Reaction Mechanism With H2SO4
Organic Chemistry | Alcohol, Ether & Epoxide Practice Problems
Reactions of Alcohols - Dehydration and Oxidation #science #chemistry #reactionsofalcohols
Reactions of Alcohols with Examples
What Are Alcohols? | Organic Chemistry | Chemistry | FuseSchool
Converting a Ketone into an Alcohol Using a Grignard Reaction #organicchemistry
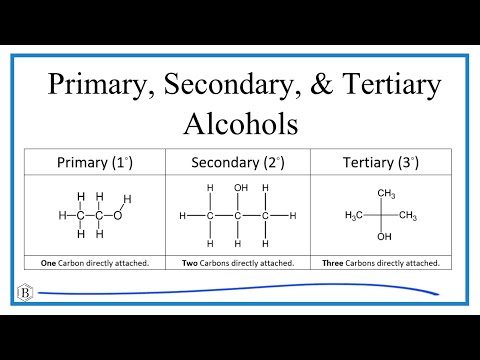
Primary, Secondary, and Tertiary Alcohols: Classification, Examples, & Practice
Grignard Reagent Reaction Mechanism
Alcohol properties | Alcohols, ethers, epoxides, sulfides | Organic chemistry | Khan Academy
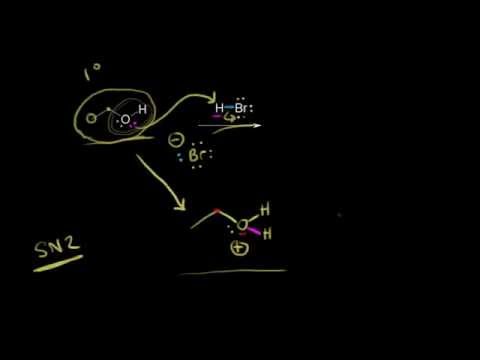
SN1 and SN2 reactions of alcohols | AOrganic chemistry | Khan Academy
Alcohol Reactions (Live Recording) Organic Chemistry Practice & Review
Grignard to Alcohol Synthesis Shortcuts - Aldehyde, Ketone, Ester
Комментарии
 0:16:07
0:16:07
 0:41:31
0:41:31
 0:04:08
0:04:08
 0:12:30
0:12:30
 0:07:39
0:07:39
 0:22:06
0:22:06
 0:54:38
0:54:38
 0:21:51
0:21:51
 0:00:41
0:00:41
 0:06:51
0:06:51
 0:16:34
0:16:34
 0:10:42
0:10:42
 0:16:59
0:16:59
 0:27:43
0:27:43
 0:11:30
0:11:30
 0:10:44
0:10:44
 0:04:03
0:04:03
 0:00:14
0:00:14
 0:03:45
0:03:45
 0:12:50
0:12:50
 0:09:24
0:09:24
 0:10:23
0:10:23
 0:58:13
0:58:13
 0:07:23
0:07:23