filmov
tv
Noble Gases

Показать описание
noble gas, any of the seven chemical elements that make up Group 18 (VIIIa) of the periodic table. The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og).
The noble gases are colourless, odourless, tasteless, nonflammable gases. They traditionally have been labeled Group 0 in the periodic table because for decades after their discovery it was believed that they could not bond to other atoms; that is, that their atoms could not combine with those of other elements to form chemical compounds. Their electronic structures and the finding that some of them do indeed form compounds has led to the more appropriate designation, Group 18.
periodic table
noble gas. noun. no·ble gas ˈnō-bəl- : any of a group of rare gases that include helium, neon, argon, krypton, xenon, and sometimes radon and that exhibit great stability and extremely low reaction rates. called also inert gas
Group 8A (or VIIIA) of the periodic table are the noble gases or inert gases: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). The name comes from the fact that these elements are virtually unreactive towards other elements or compounds
Uranium is the heaviest naturally occurring element on Earth.
Helium, neon, argon, krypton, xenon, radon, and oganesson are the seven noble gases. The least reactive chemical elements are noble gases. Because the atoms ...
Helium, neon, argon, krypton, xenon, radon, and oganesson are the seven noble gases. The least reactive chemical elements are noble gases. Because the atoms ...
नोबल गैस। संज्ञा। नो · ब्ली गैस नो-बेल-: दुर्लभ गैसों के समूह में से कोई भी जिसमें हीलियम, नियॉन, आर्गन, क्रिप्टन, क्सीनन और कभी-कभी रेडॉन शामिल हैं और जो बहुत स्थिरता और बेहद कम प्रतिक्रिया दर प्रदर्शित करते हैं। अक्रिय गैस भी कहते हैं।
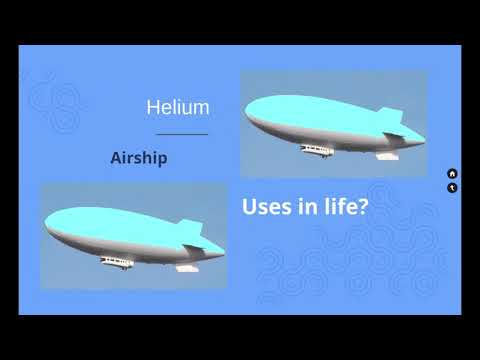
The noble gases are used to form inert atmospheres, typically for arc welding, to protect specimens, and to deter chemical reactions. The elements are used in lamps, such as neon lights and krypton headlamps, and in lasers. Helium is used in balloons, for deep-sea diving air tanks, and to cool superconducting magnets.
Krypton is named from the Greek word kryptos, “hidden.” When a current of electricity is passed through a glass tube containing krypton at low pressure, a bluish white light is emitted.
आवर्त सारणी के सबसे दाहिने स्तंभ में छह महान गैसें पाई जाती हैं। हीलियम (He) नाम की उत्पत्ति ग्रीक हेलिओस से हुई है, जिसका अर्थ है सूर्य; नियोन (ने) ग्रीक नियोस से बना है, जिसका अर्थ है नया। आर्गन (Ar) ग्रीक आर्गन से निकला है, जिसका अर्थ है निष्क्रिय; क्रिप्टन (Kr) ग्रीक क्रिप्टोस से, जिसका अर्थ छिपा हुआ है।
periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus.15-May-
Why did Ncert remove the periodic table?
Periodic table, evolution removed: NCERT said that a “considered opinion” emerged, based on the feedback of various stakeholders, including teachers, that children might not have to study certain concepts at different stages but only at an appropriate stage.10
Scientists use the periodic table to quickly refer to information about an element, like atomic mass and chemical symbol. The periodic table's arrangement also allows scientists to discern trends in element properties, including electronegativity, ionization energy, and atomic radius.
The name is the Anglo-Saxon word for the metal and the symbol comes from the Latin 'aurum', gold.
How to learn periodic table 1 to 30?
If we are talking about the first 30 elements then the periodic table starts with Hydrogen and ends at Zinc that is an element with atomic number 30. These elements can be remembered by this line: Harley Health Like Beautiful Body of Cheetah Name Opposite Falcon Nest
How do you find Valency?
The electrons in the outermost shell of an atom are called valence electrons. For elements having one to four valence electrons, valency = valence electrons. For elements having five to seven valence electrons, valency = (8-valence electrons)
Elements with a valency of 1 can either be metals with 1 valence electron (Li, Na, K, Rb, Cs) or nonmetals with 7 valence electrons (F, Cl, Br, I).
Rajiv Tasham
RT TUITION CENTRE
rt academy
by rajiv tasham
motivation9info
viral videos
new songs
chemical Reactions
The noble gases are colourless, odourless, tasteless, nonflammable gases. They traditionally have been labeled Group 0 in the periodic table because for decades after their discovery it was believed that they could not bond to other atoms; that is, that their atoms could not combine with those of other elements to form chemical compounds. Their electronic structures and the finding that some of them do indeed form compounds has led to the more appropriate designation, Group 18.
periodic table
noble gas. noun. no·ble gas ˈnō-bəl- : any of a group of rare gases that include helium, neon, argon, krypton, xenon, and sometimes radon and that exhibit great stability and extremely low reaction rates. called also inert gas
Group 8A (or VIIIA) of the periodic table are the noble gases or inert gases: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). The name comes from the fact that these elements are virtually unreactive towards other elements or compounds
Uranium is the heaviest naturally occurring element on Earth.
Helium, neon, argon, krypton, xenon, radon, and oganesson are the seven noble gases. The least reactive chemical elements are noble gases. Because the atoms ...
Helium, neon, argon, krypton, xenon, radon, and oganesson are the seven noble gases. The least reactive chemical elements are noble gases. Because the atoms ...
नोबल गैस। संज्ञा। नो · ब्ली गैस नो-बेल-: दुर्लभ गैसों के समूह में से कोई भी जिसमें हीलियम, नियॉन, आर्गन, क्रिप्टन, क्सीनन और कभी-कभी रेडॉन शामिल हैं और जो बहुत स्थिरता और बेहद कम प्रतिक्रिया दर प्रदर्शित करते हैं। अक्रिय गैस भी कहते हैं।
The noble gases are used to form inert atmospheres, typically for arc welding, to protect specimens, and to deter chemical reactions. The elements are used in lamps, such as neon lights and krypton headlamps, and in lasers. Helium is used in balloons, for deep-sea diving air tanks, and to cool superconducting magnets.
Krypton is named from the Greek word kryptos, “hidden.” When a current of electricity is passed through a glass tube containing krypton at low pressure, a bluish white light is emitted.
आवर्त सारणी के सबसे दाहिने स्तंभ में छह महान गैसें पाई जाती हैं। हीलियम (He) नाम की उत्पत्ति ग्रीक हेलिओस से हुई है, जिसका अर्थ है सूर्य; नियोन (ने) ग्रीक नियोस से बना है, जिसका अर्थ है नया। आर्गन (Ar) ग्रीक आर्गन से निकला है, जिसका अर्थ है निष्क्रिय; क्रिप्टन (Kr) ग्रीक क्रिप्टोस से, जिसका अर्थ छिपा हुआ है।
periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus.15-May-
Why did Ncert remove the periodic table?
Periodic table, evolution removed: NCERT said that a “considered opinion” emerged, based on the feedback of various stakeholders, including teachers, that children might not have to study certain concepts at different stages but only at an appropriate stage.10
Scientists use the periodic table to quickly refer to information about an element, like atomic mass and chemical symbol. The periodic table's arrangement also allows scientists to discern trends in element properties, including electronegativity, ionization energy, and atomic radius.
The name is the Anglo-Saxon word for the metal and the symbol comes from the Latin 'aurum', gold.
How to learn periodic table 1 to 30?
If we are talking about the first 30 elements then the periodic table starts with Hydrogen and ends at Zinc that is an element with atomic number 30. These elements can be remembered by this line: Harley Health Like Beautiful Body of Cheetah Name Opposite Falcon Nest
How do you find Valency?
The electrons in the outermost shell of an atom are called valence electrons. For elements having one to four valence electrons, valency = valence electrons. For elements having five to seven valence electrons, valency = (8-valence electrons)
Elements with a valency of 1 can either be metals with 1 valence electron (Li, Na, K, Rb, Cs) or nonmetals with 7 valence electrons (F, Cl, Br, I).
Rajiv Tasham
RT TUITION CENTRE
rt academy
by rajiv tasham
motivation9info
viral videos
new songs
chemical Reactions
 0:04:12
0:04:12
 0:04:49
0:04:49
 0:11:49
0:11:49
 0:22:26
0:22:26
 0:07:30
0:07:30
 0:08:59
0:08:59
 0:00:14
0:00:14
 0:02:46
0:02:46
 0:00:11
0:00:11
 0:02:32
0:02:32
 0:01:01
0:01:01
 0:04:44
0:04:44
 0:10:06
0:10:06
 0:00:54
0:00:54
 0:06:47
0:06:47
 0:00:07
0:00:07
 0:28:28
0:28:28
 0:03:56
0:03:56
 0:03:00
0:03:00
 0:03:04
0:03:04
 0:08:57
0:08:57
 0:00:29
0:00:29
 0:00:52
0:00:52
 0:00:09
0:00:09