filmov
tv
Calculate the work done on air and heat supplied [Problem 3.11] Applied Thermodynamics by McConkey

Показать описание
Calculate the work done on the air and heat supplied [Problem 3.11] Applied Thermodynamics by McConkey
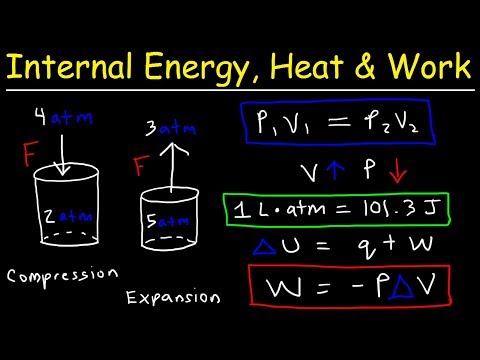
Problem 3.11: 1 kg of air at 1.02 bar, 20 C is compressed reversibly according to a law PV^1.3 = Constant, to a pressure of 5.5 bar. Calculate the work done on the air and the heat supplied during the compression. Problem (3.11),
Work done on the air,
Heat supplied,
Specific gas constant R,
increase in internal energy,
Specific heat capacity at constant volume,
Specific heat capacity at constant Pressure,
mass of gas,
Work Input,
Heat Supplied,
final temperature of oxygen,
Idle gas equation,
Perfect gas equation,
Molar mass,
Molar gas constant,
McConkey,
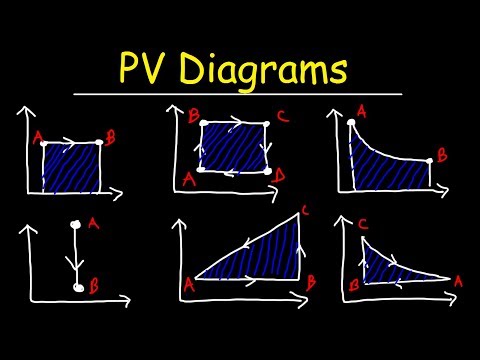
PV diagram,
Superheating,
Saturated Steam Tab,
Superheating Steam Table
Wet Region,
Superheated Region,
mass of steam,
dry saturated,
Problems,
Solution of Problems,
Solution of Thermodynamics Problems,
Reversibility,
Degree of Superheat,
Saturated Temperature,
Superheated Temperature,
Saturated Liquid Line,
Saturated Vapour Line,
Thermodynamic cycle,
Applied Thermodynamics,
McConkey,
Caculate state of Internal energy,
Gain,
Loss,
Work dome,
Final volume, final pressure,
heat rejected to the cooling water,
Velocity of the fluid at exit,
Rate of flow of fluid,
exit area of te nozzle,
Problem 3.11: 1 kg of air at 1.02 bar, 20 C is compressed reversibly according to a law PV^1.3 = Constant, to a pressure of 5.5 bar. Calculate the work done on the air and the heat supplied during the compression. Problem (3.11),
Work done on the air,
Heat supplied,
Specific gas constant R,
increase in internal energy,
Specific heat capacity at constant volume,
Specific heat capacity at constant Pressure,
mass of gas,
Work Input,
Heat Supplied,
final temperature of oxygen,
Idle gas equation,
Perfect gas equation,
Molar mass,
Molar gas constant,
McConkey,
PV diagram,
Superheating,
Saturated Steam Tab,
Superheating Steam Table
Wet Region,
Superheated Region,
mass of steam,
dry saturated,
Problems,
Solution of Problems,
Solution of Thermodynamics Problems,
Reversibility,
Degree of Superheat,
Saturated Temperature,
Superheated Temperature,
Saturated Liquid Line,
Saturated Vapour Line,
Thermodynamic cycle,
Applied Thermodynamics,
McConkey,
Caculate state of Internal energy,
Gain,
Loss,
Work dome,
Final volume, final pressure,
heat rejected to the cooling water,
Velocity of the fluid at exit,
Rate of flow of fluid,
exit area of te nozzle,
 0:03:48
0:03:48
 0:40:15
0:40:15
 0:03:54
0:03:54
 0:08:54
0:08:54
 0:04:57
0:04:57
 0:03:50
0:03:50
 0:25:08
0:25:08
 0:20:17
0:20:17
 0:45:16
0:45:16
 0:03:32
0:03:32
 0:01:22
0:01:22
 0:03:55
0:03:55
 0:23:29
0:23:29
 0:03:54
0:03:54
 0:01:59
0:01:59
 0:32:06
0:32:06
 0:11:17
0:11:17
 0:07:24
0:07:24
 0:08:32
0:08:32
 0:07:21
0:07:21
 0:03:34
0:03:34
 0:09:05
0:09:05
 0:11:32
0:11:32
 0:11:23
0:11:23