filmov
tv
Types of chemical bonding and VSEPR theory (Chemical Bonding #1)

Показать описание
LESSON OBJECTIVE: Understand how ionic, covalent and metallic bonds form. Rationalise molecular geometries using VSEPR theory.
In this lesson we give an overview of the three types of chemical bonding (ionic, covalent and metallic) and an introduction into how VSEPR theory dictates molecular geometries. This is lesson eight in our physical chemistry series for Unit 3: Chemical Bonding (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
Download the slides and student led tasks for this lesson here:
Learning Outcomes (from the Cambridge AS Chemistry Curriculum 2019-2021):
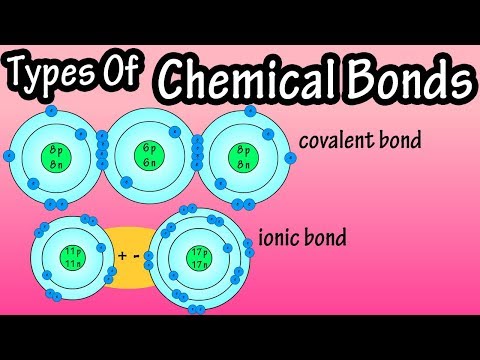
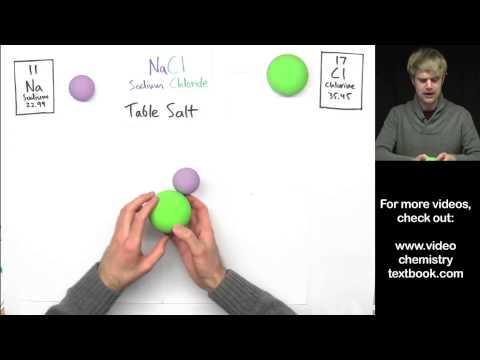
3.1 Ionic bonding
a) describe ionic bonding, using the examples of sodium chloride, magnesium oxide and calcium fluoride, including the use of ‘dot-and- cross’ diagrams [skip to 4:20]
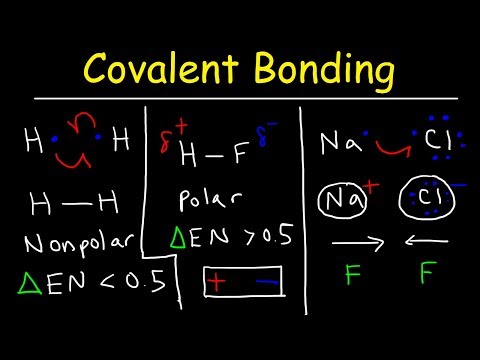
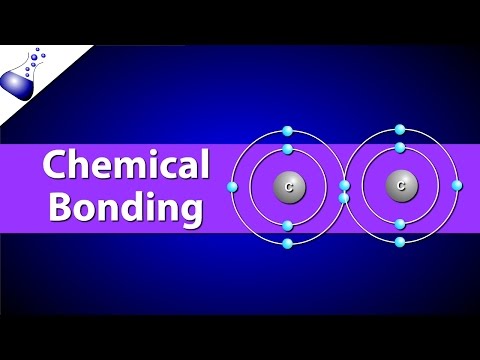
3.2 Covalent bonding and co-ordinate (dative covalent) bonding including shapes of simple molecules
a) describe, including the use of ‘dot-and-cross’ diagrams:
(i) covalent bonding, in molecules such as hydrogen, oxygen, chlorine, hydrogen chloride, carbon dioxide, methane, ethene [skip to 9:59]
(ii) co-ordinate (dative covalent) bonding, such as in the formation of the ammonium ion and in the Al2Cl6 molecule [skip to 19:03]
c) explain the shapes of, and bond angles in, molecules by using the qualitative model of electron-pair repulsion (including lone pairs), using as simple examples BF3 (trigonal planar), CO2 (linear), CH4 (tetrahedral), NH3 (pyramidal), H2O (non-linear), SF6 (octahedral), PF5 (trigonal bipyramidal) [skip to 25:39]
3.4 Metallic bonding
a) describe metallic bonding in terms of positive ions surrounded by delocalised electrons [skip to 31:44]
 0:03:33
0:03:33
 0:09:46
0:09:46
 0:11:50
0:11:50
 0:04:50
0:04:50
 0:03:03
0:03:03
 0:04:18
0:04:18
 0:03:34
0:03:34
 0:06:09
0:06:09
 0:39:10
0:39:10
 0:08:50
0:08:50
 0:12:14
0:12:14
 0:04:30
0:04:30
 0:12:41
0:12:41
 0:04:12
0:04:12
 0:07:20
0:07:20
 0:21:57
0:21:57
 0:02:42
0:02:42
 0:22:15
0:22:15
 0:08:50
0:08:50
 0:02:15
0:02:15
 0:07:04
0:07:04
 0:03:31
0:03:31
 0:23:14
0:23:14
 0:47:18
0:47:18