filmov
tv
R3.2.9 Describe, using equations, the oxidation reactions of alcohols [SL IB Chemistry]

Показать описание
This and the next video cover what you need to know but
a) warm acidified dichromate is the oxidizing agent [Cr6+ (orange) Cr3+ (green)]
b) distillation allows the collection of the aldehydes (normally this would continue to react to form a carboxylic acid)
c) reflux allows the production of the carboxylic acid
a) warm acidified dichromate is the oxidizing agent [Cr6+ (orange) Cr3+ (green)]
b) distillation allows the collection of the aldehydes (normally this would continue to react to form a carboxylic acid)
c) reflux allows the production of the carboxylic acid
Linear Algebra 2.1.2 - Generalizing from R2 to R3 (2 of 2)
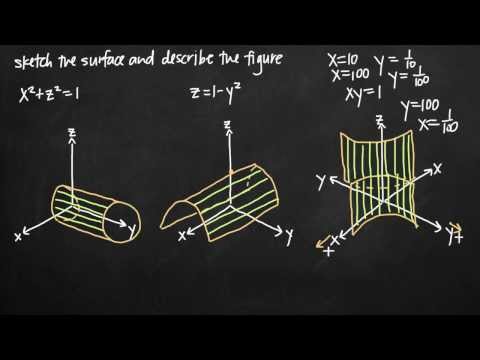
Sketching the quadric surface (KristaKingMath)
Multivariable Calculus Unit 1 Lecture 03: Five examples in R3
How To Find The Equation of a Plane Given Three Points
Solving congruences, 3 introductory examples
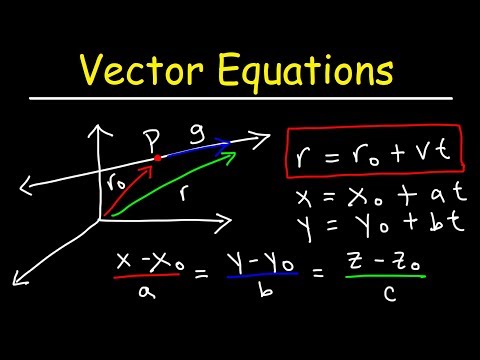
How To Find The Vector Equation of a Line and Symmetric & Parametric Equations
Line of Intersection of Two Planes
Lines in R3
How to solve system of linear equations(Week-2)
Linear Algebra Example Problems - Linear Combination of Vectors #2
How to Find the Matrix of a Linear Transformation
8.4 Intersections of Lines in R2 and R3
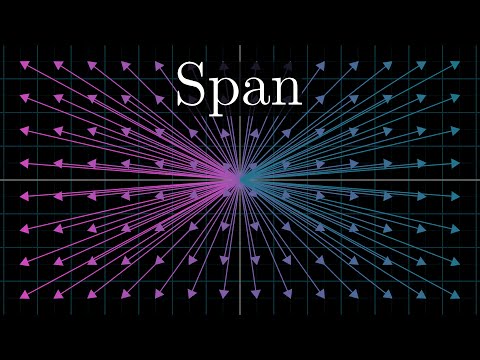
Linear combinations, span, and basis vectors | Chapter 2, Essence of linear algebra
Linear Algebra Example Problems - Spanning Vectors #1
14.1 Domain and range for multi-variable functions
How to determine if one vector is in the span of other vectors?
Solving Systems of Equations With 3 Variables & Word Problems
How to Prove a Set is a Subspace of a Vector Space
Do the Vectors Span R3? | Linear Algebra Exercises
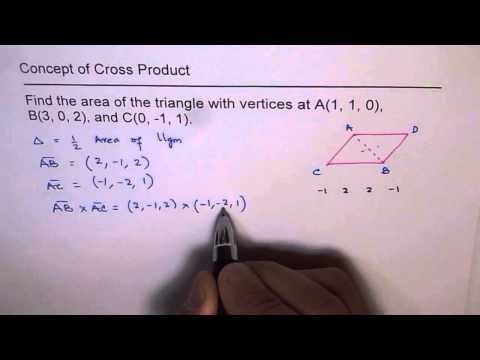
Area of Triangle with three vertices using Vector Cross Product in 3D Coordinate Plane
NECO 2020 Question 30 | Equation of a straight line
[Math 22] Lec 18 Intro to R3, distance formula, planes and spheres
Multivariable Calculus Unit 1 Lecture 02: Five examples in R3
Linear transformations and matrices | Chapter 3, Essence of linear algebra
Комментарии
 0:12:59
0:12:59
 0:08:05
0:08:05
 0:25:50
0:25:50
 0:06:56
0:06:56
 0:03:51
0:03:51
 0:11:37
0:11:37
 0:07:22
0:07:22
 0:34:18
0:34:18
 1:34:36
1:34:36
 0:03:53
0:03:53
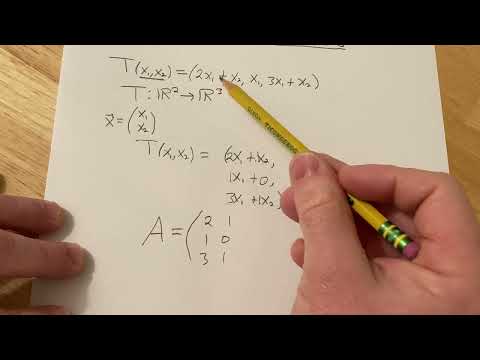 0:05:19
0:05:19
 1:04:24
1:04:24
 0:09:59
0:09:59
 0:04:31
0:04:31
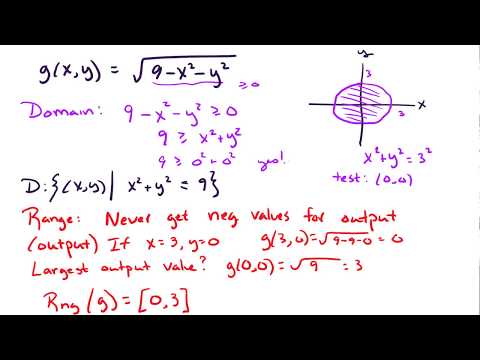 0:10:45
0:10:45
 0:05:00
0:05:00
 0:12:44
0:12:44
 0:05:58
0:05:58
 0:07:30
0:07:30
 0:06:10
0:06:10
 0:05:52
0:05:52
![[Math 22] Lec](https://i.ytimg.com/vi/3GtfzBQRLbA/hqdefault.jpg) 0:43:36
0:43:36
 0:13:45
0:13:45
 0:10:59
0:10:59