filmov
tv
Metals and Nonmetals Class 8 Science - Physical Properties of Metals and Nonmetals
Показать описание
Metals and Nonmetals Class 8 Science Chapter 4 - Physical Properties of Metals and Nonmetals
During classification of elements scientist considered few properties that differentiate between metals and nonmetals. Here are the physical properties of metals and non metals –
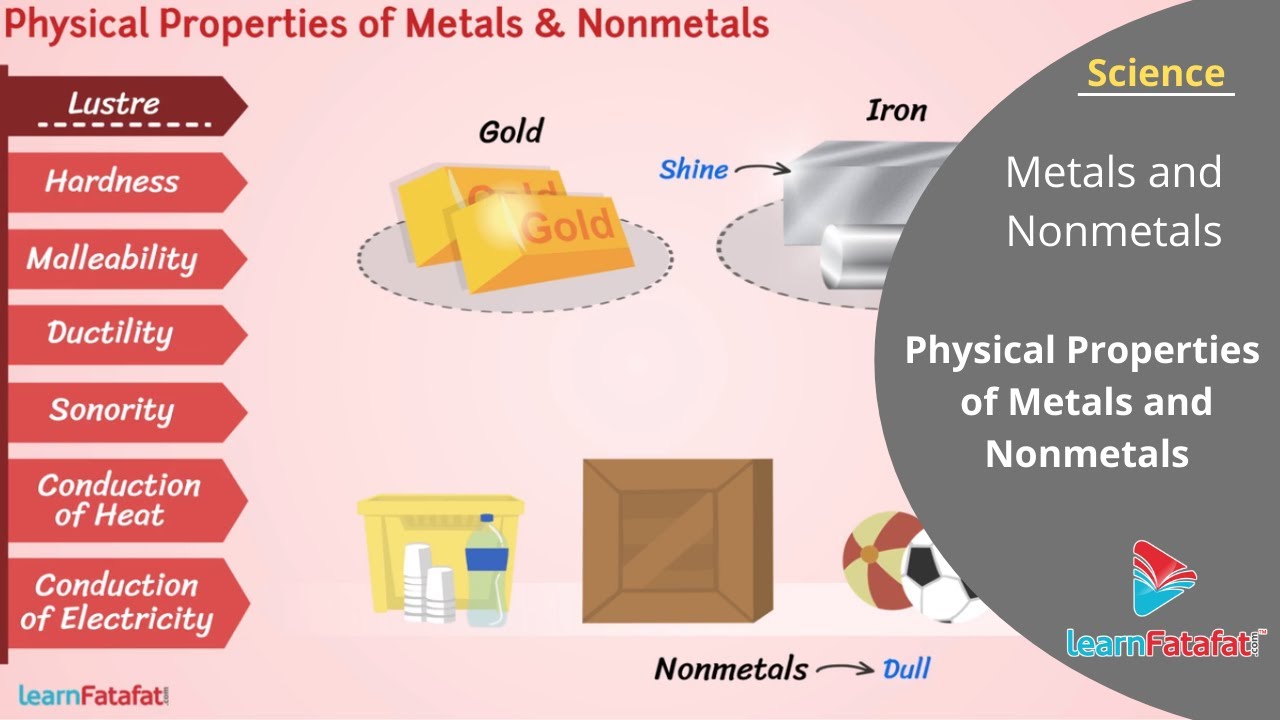
Lustre:
In pure state all metals have shining surface. This property is called metallic lustre. Nonmetals on the other hand, are dull therefore does not have lustre.
Hardness:
The hardness varies from metal to metal. On the other hand nonmetals can be cut or broken easily. So, Comparing metals and nonmetals you can find that nonmetals are soft while metals are hard.
Malleability:
Iron nail and aluminium wire change their shape but coal piece and pencil lead break into pieces. Harder we beat greater is the change of shape. Thus metals if beaten change their shape and forms thin sheet on beating. This property is called malleability. Comparing metals and nonmetals you can find that nonmetals can’t form thin sheet by beating, instead they undergo breaking.
Ductility:
Another interesting property of metals is the ability to form thin wires, it is called ductility. With this property you can see wires of aluminium, copper, iron. Nonmetals does not show this property and breaks if we try to form wires.
Sonority: The property of producing ringing sound when a substance is struck by hard substance is called sonority. Metals are sonorous but nonmetals are not.
Conduction of heat:
If one end of iron rod is heated then we can feel rise in temperature at other end also. This means that metals conduct heat. Nonmetals does not conduct heat.
Conduction of electricity:
Metals are good conductor of electricity while nonmetals are insulators.
Exceptions:
Sodium and potassium are soft and can be easily cleaved i.e. cut to form plane surface by knife however these belongs to metals. Similarly, all metals are solid except mercury which exist in liquid form. Iodine is a non-metal but it is lustrous. Diamond is hardest substance which is a nonmetal.
During classification of elements scientist considered few properties that differentiate between metals and nonmetals. Here are the physical properties of metals and non metals –
Lustre:
In pure state all metals have shining surface. This property is called metallic lustre. Nonmetals on the other hand, are dull therefore does not have lustre.
Hardness:
The hardness varies from metal to metal. On the other hand nonmetals can be cut or broken easily. So, Comparing metals and nonmetals you can find that nonmetals are soft while metals are hard.
Malleability:
Iron nail and aluminium wire change their shape but coal piece and pencil lead break into pieces. Harder we beat greater is the change of shape. Thus metals if beaten change their shape and forms thin sheet on beating. This property is called malleability. Comparing metals and nonmetals you can find that nonmetals can’t form thin sheet by beating, instead they undergo breaking.
Ductility:
Another interesting property of metals is the ability to form thin wires, it is called ductility. With this property you can see wires of aluminium, copper, iron. Nonmetals does not show this property and breaks if we try to form wires.
Sonority: The property of producing ringing sound when a substance is struck by hard substance is called sonority. Metals are sonorous but nonmetals are not.
Conduction of heat:
If one end of iron rod is heated then we can feel rise in temperature at other end also. This means that metals conduct heat. Nonmetals does not conduct heat.
Conduction of electricity:
Metals are good conductor of electricity while nonmetals are insulators.
Exceptions:
Sodium and potassium are soft and can be easily cleaved i.e. cut to form plane surface by knife however these belongs to metals. Similarly, all metals are solid except mercury which exist in liquid form. Iodine is a non-metal but it is lustrous. Diamond is hardest substance which is a nonmetal.
Комментарии
 0:09:28
0:09:28
 0:20:29
0:20:29
 0:44:29
0:44:29
 0:09:46
0:09:46
 0:04:45
0:04:45
 1:24:02
1:24:02
 0:24:43
0:24:43
 0:03:26
0:03:26
 0:47:11
0:47:11
 0:20:08
0:20:08
 0:02:59
0:02:59
 0:46:59
0:46:59
 0:20:32
0:20:32
 0:05:51
0:05:51
 0:12:28
0:12:28
 0:14:56
0:14:56
 0:21:22
0:21:22
 0:06:50
0:06:50
 2:03:03
2:03:03
 0:14:41
0:14:41
 0:49:08
0:49:08
 0:14:01
0:14:01
 2:22:33
2:22:33
 8:02:53
8:02:53