filmov
tv
General Chemistry 1A. Lecture 01. Introduction to General Chemistry.

Показать описание
UCI Chem 1A General Chemistry (Winter 2013)
Lec 01. General Chemistry Introduction to General Chemistry
Instructor: Amanda Brindley, Ph.D.
License: Creative Commons BY-NC-SA
Description: Chem 1A is the first quarter of General Chemistry and covers the following topics: atomic structure; general properties of the elements; covalent, ionic, and metallic bonding; intermolecular forces; mass relationships.
This video is part of a 23-lecture undergraduate-level course titled "General Chemistry" taught at UC Irvine by Amanda Brindley, Ph.D.
Recorded February 25, 2013.
Index of Topics:
0:01:55 "Fundamentals"
0:02:40 Significant Figures
0:11:44 Dimensional Analysis: Conversion Factors
0:12:30 Fundamental Problems
0:22:22 Structure of an Atom
0:25:07 Cartoon
0:26:06 Isotopes, Natural abundance, and Molecular Mass
0:29:11 Average Molecular Mass
0:32:29 Periodic Table
0:34:21 Naming
0:37:12 Bonding
0:38:09 Types of Bonding
0:40:28 Empirical vs. Chemical Formulas
0:42:30 Cartoon: Ionic Bonds
0:43:28 Ionic Bonds
0:48:26 Charges of Atoms
0:50:50 Inert Pair Effect
0:51:36 Naming Ionic Compounds
Lec 01. General Chemistry Introduction to General Chemistry
Instructor: Amanda Brindley, Ph.D.
License: Creative Commons BY-NC-SA
Description: Chem 1A is the first quarter of General Chemistry and covers the following topics: atomic structure; general properties of the elements; covalent, ionic, and metallic bonding; intermolecular forces; mass relationships.
This video is part of a 23-lecture undergraduate-level course titled "General Chemistry" taught at UC Irvine by Amanda Brindley, Ph.D.
Recorded February 25, 2013.
Index of Topics:
0:01:55 "Fundamentals"
0:02:40 Significant Figures
0:11:44 Dimensional Analysis: Conversion Factors
0:12:30 Fundamental Problems
0:22:22 Structure of an Atom
0:25:07 Cartoon
0:26:06 Isotopes, Natural abundance, and Molecular Mass
0:29:11 Average Molecular Mass
0:32:29 Periodic Table
0:34:21 Naming
0:37:12 Bonding
0:38:09 Types of Bonding
0:40:28 Empirical vs. Chemical Formulas
0:42:30 Cartoon: Ionic Bonds
0:43:28 Ionic Bonds
0:48:26 Charges of Atoms
0:50:50 Inert Pair Effect
0:51:36 Naming Ionic Compounds
Комментарии
 0:55:55
0:55:55
 0:54:48
0:54:48
 1:07:21
1:07:21
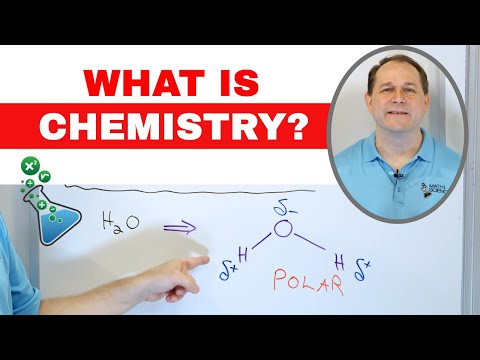 1:08:40
1:08:40
 0:25:12
0:25:12
 0:41:20
0:41:20
 0:07:45
0:07:45
 0:31:15
0:31:15
 0:21:19
0:21:19
 0:07:31
0:07:31
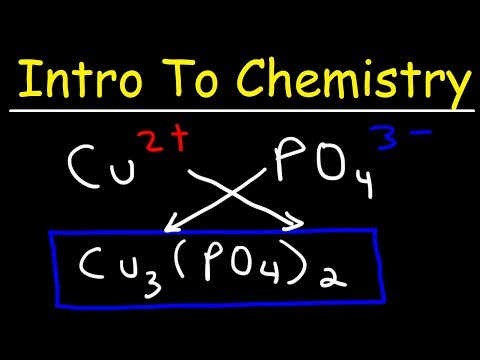 0:52:09
0:52:09
 0:48:26
0:48:26
 0:48:54
0:48:54
 0:19:11
0:19:11
 0:05:30
0:05:30
 1:10:34
1:10:34
 0:22:15
0:22:15
 0:37:29
0:37:29
 0:33:19
0:33:19
 0:55:17
0:55:17
 0:26:28
0:26:28
 0:19:51
0:19:51
 0:18:14
0:18:14
 0:00:30
0:00:30