filmov
tv
Nuclear Chemistry: Comparing & Detecting Ionizing Radiation (α β γ) and Balancing Nuclear Reactions

Показать описание
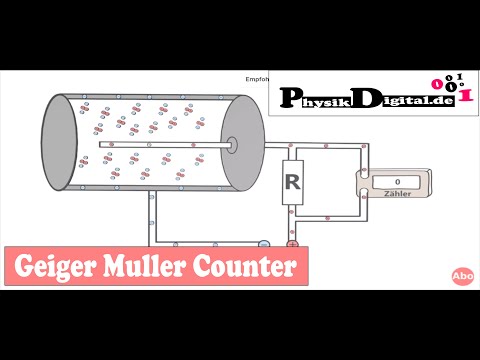
Ketzbook describes nuclear decay and specifically looks at alpha, beta, and gamma radiation. They can distinguished by their behavior in an electric or magnetic field, their penetrating ability, and their ionizing strength. Nuclear radiation is dangerous to the body and can be detected numerous ways. Alpha and beta decay cause a change in the type of element in a predictable way. In a nuclear reaction, the mass number and the nuclear charge are preserved (balanced).
Check out this example of a cloud chamber:
If you are wondering, yes I know about neutrinos, and like most high school level chemistry courses, I do not think it is appropriate to discuss neutrinos here.
Also, if you feel like my quality of videos has decreased, you are correct. Because of COVID-19, I have had to produce a lot of material in a small amount of time. Making a polished animated video would take me about 30 times as long as it took to produce this video. I WILL continue to make animated polished videos in the future, so don't worry. Thank you for your support!
#nuclear #nuclearRadiation #nuclearReactions #madeEasy #ketzbook #tutorial #COVID
Check out this example of a cloud chamber:
If you are wondering, yes I know about neutrinos, and like most high school level chemistry courses, I do not think it is appropriate to discuss neutrinos here.
Also, if you feel like my quality of videos has decreased, you are correct. Because of COVID-19, I have had to produce a lot of material in a small amount of time. Making a polished animated video would take me about 30 times as long as it took to produce this video. I WILL continue to make animated polished videos in the future, so don't worry. Thank you for your support!
#nuclear #nuclearRadiation #nuclearReactions #madeEasy #ketzbook #tutorial #COVID
Комментарии
 0:28:28
0:28:28
 0:06:43
0:06:43
 0:00:21
0:00:21
 0:09:38
0:09:38
 0:06:18
0:06:18
 0:00:56
0:00:56
 0:29:56
0:29:56
 0:00:31
0:00:31
 0:00:37
0:00:37
 0:06:34
0:06:34
 0:02:38
0:02:38
 0:00:35
0:00:35
 0:09:32
0:09:32
 0:12:51
0:12:51
 0:02:22
0:02:22
 0:39:08
0:39:08
 0:00:59
0:00:59
 0:33:28
0:33:28
 0:24:15
0:24:15
 0:14:23
0:14:23
 0:00:14
0:00:14
 0:04:47
0:04:47
 0:54:33
0:54:33
 0:46:32
0:46:32