filmov
tv
Surface Tension - What is it, how does it form, what properties does it impart

Показать описание
How does surface tension affect the surface properties of a liquid? Looking at surface tension from a particle perspective and a macro perspective, this video shows what causes surface tension, how surface tension is manifested in our everyday lives, and how intermolecular forces are involved in surface tension.
—More on Surface tension | Wikipedia— 1/5/2018:
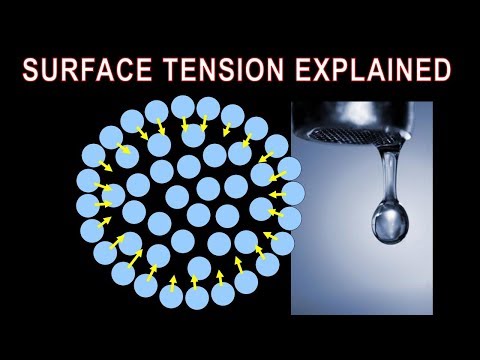
Surface tension is the elastic tendency of a fluid surface which makes it acquire the least surface area possible. Surface tension allows insects (e.g. water striders), usually denser than water, to float and stride on a water surface.
At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion). The net effect is an inward force at its surface that causes the liquid to behave as if its surface were covered with a stretched elastic membrane. Thus, the surface becomes under tension from the imbalanced forces, which is probably where the term "surface tension" came from.[1] Because of the relatively high attraction of water molecules for each other through a web of hydrogen bonds, water has a higher surface tension (72.8 millinewtons per meter at 20 °C) compared to that of most other liquids. Surface tension is an important factor in the phenomenon of capillarity.
Surface tension has the dimension of force per unit length, or of energy per unit area. The two are equivalent, but when referring to energy per unit of area, it is common to use the term surface energy, which is a more general term in the sense that it applies also to solids.
In materials science, surface tension is used for either surface stress or surface free energy.
Water[edit]
Several effects of surface tension can be seen with ordinary water:
Beading of rain water on a waxy surface, such as a leaf. Water adheres weakly to wax and strongly to itself, so water clusters into drops. Surface tension gives them their near-spherical shape, because a sphere has the smallest possible surface area to volume ratio.
Formation of drops occurs when a mass of liquid is stretched. The animation (below) shows water adhering to the faucet gaining mass until it is stretched to a point where the surface tension can no longer keep the drop linked to the faucet. It then separates and surface tension forms the drop into a sphere. If a stream of water was running from the faucet, the stream would break up into drops during its fall. Gravity stretches the stream, then surface tension pinches it into spheres.[3]
Flotation of objects denser than water occurs when the object is nonwettable and its weight is small enough to be borne by the forces arising from surface tension.[2] For example, water striders use surface tension to walk on the surface of a pond in the following way. The nonwettability of the water strider's leg means there is no attraction between molecules of the leg and molecules of the water, so when the leg pushes down on the water, the surface tension of the water only tries to recover its flatness from its deformation due to the leg. This behavior of the water pushes the water strider upward so it can stand on the surface of the water as long as its mass is small enough that the water can support it. The surface of the water behaves like an elastic film: the insect's feet cause indentations in the water's surface, increasing its surface area[4] and tendency of minimization of surface curvature (so area) of the water pushes the insect's feet upward.
Separation of oil and water (in this case, water and liquid wax) is caused by a tension in the surface between dissimilar liquids. This type of surface tension is called "interface tension", but its chemistry is the same.
Tears of wine is the formation of drops and rivulets on the side of a glass containing an alcoholic beverage. Its cause is a complex interaction between the differing surface tensions of water and ethanol; it is induced by a combination of surface tension modification of water by ethanol together with ethanol evaporating faster than water.
Check out other popular CC Academy videos on this channel:
Stoichiometry Tutorial, step by step
Classifying Types of Chemical Reactions
Solution Stoichiometry
Orbitals, the Basics: Atomic Orbitals Tutorial
Hybrid Orbitals Explained
Polar Molecules Tutorial: How to determine polarity in a molecule
Metallic Bonding and Metallic Properties Explained
Covalent Bonding Tutorial
Ionic Bonds, Ionic Compounds: What is an ionic bond and how do ionic compounds form
Electronegativity and bond character (bond type): non-polar covalent, polar, ionic
Metric Unit Prefix Conversions
Metric unit conversions shortcut
Mole Conversions Tutorial
Frequency, Wavelength, and the Speed of Light
The Bohr Model of the Atom and Atomic Emission Spectra
What is Heat
Rutherford's Gold Foil Experiment
Unit Conversion Using Dimensional Analysis Tutorial
What is Fire: Combustion Reaction Tutorial
—More on Surface tension | Wikipedia— 1/5/2018:
Surface tension is the elastic tendency of a fluid surface which makes it acquire the least surface area possible. Surface tension allows insects (e.g. water striders), usually denser than water, to float and stride on a water surface.
At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to cohesion) than to the molecules in the air (due to adhesion). The net effect is an inward force at its surface that causes the liquid to behave as if its surface were covered with a stretched elastic membrane. Thus, the surface becomes under tension from the imbalanced forces, which is probably where the term "surface tension" came from.[1] Because of the relatively high attraction of water molecules for each other through a web of hydrogen bonds, water has a higher surface tension (72.8 millinewtons per meter at 20 °C) compared to that of most other liquids. Surface tension is an important factor in the phenomenon of capillarity.
Surface tension has the dimension of force per unit length, or of energy per unit area. The two are equivalent, but when referring to energy per unit of area, it is common to use the term surface energy, which is a more general term in the sense that it applies also to solids.
In materials science, surface tension is used for either surface stress or surface free energy.
Water[edit]
Several effects of surface tension can be seen with ordinary water:
Beading of rain water on a waxy surface, such as a leaf. Water adheres weakly to wax and strongly to itself, so water clusters into drops. Surface tension gives them their near-spherical shape, because a sphere has the smallest possible surface area to volume ratio.
Formation of drops occurs when a mass of liquid is stretched. The animation (below) shows water adhering to the faucet gaining mass until it is stretched to a point where the surface tension can no longer keep the drop linked to the faucet. It then separates and surface tension forms the drop into a sphere. If a stream of water was running from the faucet, the stream would break up into drops during its fall. Gravity stretches the stream, then surface tension pinches it into spheres.[3]
Flotation of objects denser than water occurs when the object is nonwettable and its weight is small enough to be borne by the forces arising from surface tension.[2] For example, water striders use surface tension to walk on the surface of a pond in the following way. The nonwettability of the water strider's leg means there is no attraction between molecules of the leg and molecules of the water, so when the leg pushes down on the water, the surface tension of the water only tries to recover its flatness from its deformation due to the leg. This behavior of the water pushes the water strider upward so it can stand on the surface of the water as long as its mass is small enough that the water can support it. The surface of the water behaves like an elastic film: the insect's feet cause indentations in the water's surface, increasing its surface area[4] and tendency of minimization of surface curvature (so area) of the water pushes the insect's feet upward.
Separation of oil and water (in this case, water and liquid wax) is caused by a tension in the surface between dissimilar liquids. This type of surface tension is called "interface tension", but its chemistry is the same.
Tears of wine is the formation of drops and rivulets on the side of a glass containing an alcoholic beverage. Its cause is a complex interaction between the differing surface tensions of water and ethanol; it is induced by a combination of surface tension modification of water by ethanol together with ethanol evaporating faster than water.
Check out other popular CC Academy videos on this channel:
Stoichiometry Tutorial, step by step
Classifying Types of Chemical Reactions
Solution Stoichiometry
Orbitals, the Basics: Atomic Orbitals Tutorial
Hybrid Orbitals Explained
Polar Molecules Tutorial: How to determine polarity in a molecule
Metallic Bonding and Metallic Properties Explained
Covalent Bonding Tutorial
Ionic Bonds, Ionic Compounds: What is an ionic bond and how do ionic compounds form
Electronegativity and bond character (bond type): non-polar covalent, polar, ionic
Metric Unit Prefix Conversions
Metric unit conversions shortcut
Mole Conversions Tutorial
Frequency, Wavelength, and the Speed of Light
The Bohr Model of the Atom and Atomic Emission Spectra
What is Heat
Rutherford's Gold Foil Experiment
Unit Conversion Using Dimensional Analysis Tutorial
What is Fire: Combustion Reaction Tutorial
Комментарии
 0:03:11
0:03:11
 0:03:51
0:03:51
 0:06:38
0:06:38
 0:01:03
0:01:03
 0:03:37
0:03:37
 0:10:11
0:10:11
 0:12:54
0:12:54
 0:08:16
0:08:16
 0:09:23
0:09:23
 0:04:22
0:04:22
 0:04:30
0:04:30
 0:01:31
0:01:31
 0:00:59
0:00:59
 0:00:16
0:00:16
 0:00:53
0:00:53
 0:03:28
0:03:28
 0:17:35
0:17:35
 0:00:56
0:00:56
 0:01:00
0:01:00
 0:11:59
0:11:59
 0:00:32
0:00:32
 0:00:36
0:00:36
 0:00:59
0:00:59
 0:00:59
0:00:59