filmov
tv
Surface Tension of Water, Capillary Action, Cohesive and Adhesive Forces - Work & Potential Energy
Показать описание
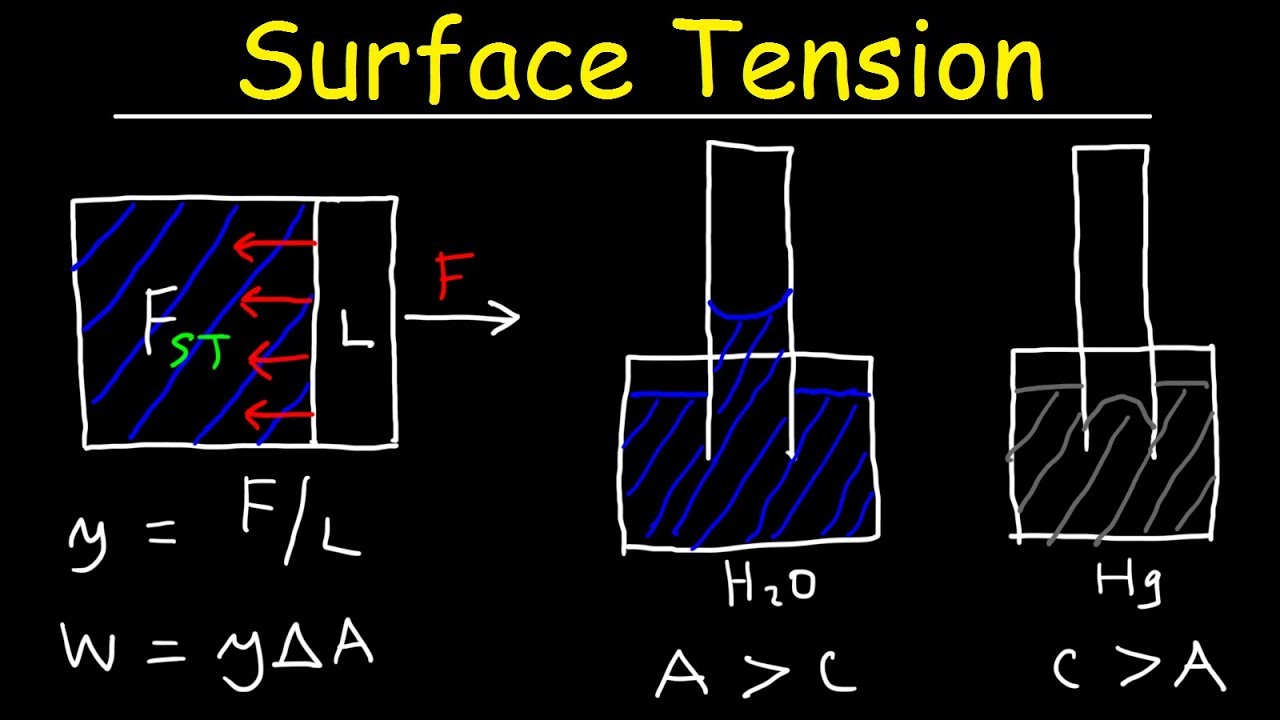
This physics video tutorial provides a basic introduction into the surface tension of water. Surface tension prevents small amounts of water from flattening out across a surface. Rather, it causes water to minimize its surface area and as a result, water forms small beadlike droplets. This video also discusses the capillary action of water as it is draw up through small tubes known as capillaries. Surface tension can be calculated by divided the force per unit length required to increase the area of a fluid. The surface tension opposes the force and attempts to minimize the area. The work required to increase the area of a fluid by a force is the surface tension multiplied by the increase in area. This video discusses the difference in the capillary effects between water and mercury. Water rises above the surrounding fluid level in a thin glass tube where as mercury descends below that line. The adhesive forces in water is greater than the cohesive forces. In Mercury, the cohesive forces are greater than the adhesive forces. Adhesive forces exist between different molecules and atoms. Cohesive forces exist between the same type of molecules and atoms.
Apparent Weight and Apparent Mass:
Fractional Volume of Submerged Object:
Hydrometer Physics Problems:
Volume Flow Rate and Mass Flow Rate:
Equation of Continuity:
Bernoulli's Equation:
________________________________
Torricelli's Theorem & Speed of Efflux:
Venturi Meter Problems:
Dynamic Lift Force:
Viscosity of Fluids and Velocity Gradient:
Poiseuille's Law:
_________________________________
Fluid Pressure Review:
Simple Harmonic Motion:
The Simple Pendulum:
Full-Length Videos and Worksheets:
Physics PDF Worksheets:
Apparent Weight and Apparent Mass:
Fractional Volume of Submerged Object:
Hydrometer Physics Problems:
Volume Flow Rate and Mass Flow Rate:
Equation of Continuity:
Bernoulli's Equation:
________________________________
Torricelli's Theorem & Speed of Efflux:
Venturi Meter Problems:
Dynamic Lift Force:
Viscosity of Fluids and Velocity Gradient:
Poiseuille's Law:
_________________________________
Fluid Pressure Review:
Simple Harmonic Motion:
The Simple Pendulum:
Full-Length Videos and Worksheets:
Physics PDF Worksheets:
Комментарии
 0:12:54
0:12:54
 0:00:30
0:00:30
 0:07:23
0:07:23
 0:00:58
0:00:58
 0:10:11
0:10:11
 0:01:48
0:01:48
 0:03:11
0:03:11
 0:06:38
0:06:38
 0:04:39
0:04:39
 0:00:54
0:00:54
 0:03:37
0:03:37
 0:03:51
0:03:51
 0:00:17
0:00:17
 0:06:58
0:06:58
 0:00:13
0:00:13
 0:00:15
0:00:15
 0:00:59
0:00:59
 0:00:41
0:00:41
 0:04:22
0:04:22
 0:03:07
0:03:07
 0:00:15
0:00:15
 0:00:16
0:00:16
 0:11:59
0:11:59
 0:02:51
0:02:51