filmov
tv
Elements and Compounds

Показать описание
RELATED VIDEOS:
ELEMENTS AND COMPOUNDS
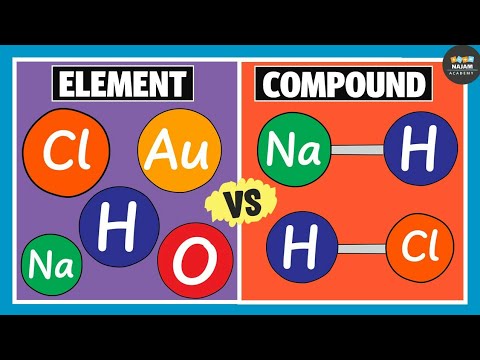
Matter can be classified as mixtures and substances. Substances can be elements and compounds.
Elements and compounds have similarities. They are both pure substances, homogeneous or have uniform compositions and made up of atoms.
ELEMENTS
Elements are the simplest form of matter. They are made up of atoms.
Atoms are the smallest particles. Atoms have 3 parts. The 3 parts of atoms are protons which are positive charge, neutrons which are no charge, and electrons which are negative charge.
Elements are logically arranged in periodic table of elements. There are 118 elements in the periodic table. Some examples are Gold commonly, used as jewelry; Helium which is the gas inside the balloons; and Mercury which can be found inside the thermometer.
Elements have unique properties. NO two elements are the same.
COMPOUNDS
When two elements are combined, they formed compound.
For example, two atoms of hydrogen which are element bonded with 1 atom of oxygen which is another element, Dihydrogen monoxide or H2O is formed. It is commonly known as water. Water is a compound.
The new substance or compound has different properties than the elements they are made of because, Hydrogen and Oxygen are both gases but they formed liquid compound. Additionally, Hydrogen is very flammable and, although oxygen is not flammable but it can make the fire to burn hotter and faster. However, the water, which is their combination kills or extinguishes fire.
Thus, compounds are made up of elements which are chemically combined. They have different properties from the elements they are made of.
DIFFERENCES BETWEEN ELEMENTS AND COMPOUNS
1. Composition
Elements are made up of one or more atoms of the same kind while compounds are made up of Two or more different kinds of atoms or elements.
2. Particle
The atoms of elements can be isolated or combined with the same atoms while the particles of compounds combined with two or more different types of elements.
3. Matter Separation
Elements cannot be divided/ broken down into simpler form while compounds can be divided/ broken down into simpler substance by chemical process.
Element is composed of one type of element so it cannot be broken down into simpler form while Compound is consisted of more than one element so it can be broken down into its component.
4. Type
The types of elements are metals, nonmetals and metalloids while the types of compounds are acids, bases and salt.
5. Examples. The examples of elements are all elements in Periodic Table and all combinations of the same kind of elements while the examples of compounds are all combinations of different kinds elements.
Specifically, the examples of elements which are metals are Iron, Gold, Silver; Nonmetals are Nitrogen, Oxygen, Hydrogen and Metalloids are Boron, Silicon and Germanium. While the specific examples of compounds are salt which is a combination of Sodium and chlorine; water which is a byproduct of hydrogen and oxygen; and rust which is formed when iron reacts with oxygen.
TAGS:
Elements
Compounds
Elements ad Compounds
Elements VS Compounds
Elements and Compounds for Grade 7
Elements and Compounds Chemistry
Elements and Compound Difference
Elements and Compounds Examples
Compound Elements Examples
Elements Chemistry
Compounds Chemistry
Elements Examples
Compounds Examples
Comparing Elements and Compounds
Elements and Compounds Class 7
Classifying Elements and Compounds
Characteristics of Elements and Compounds
Elements Compounds
ELEMENTS AND COMPOUNDS
Matter can be classified as mixtures and substances. Substances can be elements and compounds.
Elements and compounds have similarities. They are both pure substances, homogeneous or have uniform compositions and made up of atoms.
ELEMENTS
Elements are the simplest form of matter. They are made up of atoms.
Atoms are the smallest particles. Atoms have 3 parts. The 3 parts of atoms are protons which are positive charge, neutrons which are no charge, and electrons which are negative charge.
Elements are logically arranged in periodic table of elements. There are 118 elements in the periodic table. Some examples are Gold commonly, used as jewelry; Helium which is the gas inside the balloons; and Mercury which can be found inside the thermometer.
Elements have unique properties. NO two elements are the same.
COMPOUNDS
When two elements are combined, they formed compound.
For example, two atoms of hydrogen which are element bonded with 1 atom of oxygen which is another element, Dihydrogen monoxide or H2O is formed. It is commonly known as water. Water is a compound.
The new substance or compound has different properties than the elements they are made of because, Hydrogen and Oxygen are both gases but they formed liquid compound. Additionally, Hydrogen is very flammable and, although oxygen is not flammable but it can make the fire to burn hotter and faster. However, the water, which is their combination kills or extinguishes fire.
Thus, compounds are made up of elements which are chemically combined. They have different properties from the elements they are made of.
DIFFERENCES BETWEEN ELEMENTS AND COMPOUNS
1. Composition
Elements are made up of one or more atoms of the same kind while compounds are made up of Two or more different kinds of atoms or elements.
2. Particle
The atoms of elements can be isolated or combined with the same atoms while the particles of compounds combined with two or more different types of elements.
3. Matter Separation
Elements cannot be divided/ broken down into simpler form while compounds can be divided/ broken down into simpler substance by chemical process.
Element is composed of one type of element so it cannot be broken down into simpler form while Compound is consisted of more than one element so it can be broken down into its component.
4. Type
The types of elements are metals, nonmetals and metalloids while the types of compounds are acids, bases and salt.
5. Examples. The examples of elements are all elements in Periodic Table and all combinations of the same kind of elements while the examples of compounds are all combinations of different kinds elements.
Specifically, the examples of elements which are metals are Iron, Gold, Silver; Nonmetals are Nitrogen, Oxygen, Hydrogen and Metalloids are Boron, Silicon and Germanium. While the specific examples of compounds are salt which is a combination of Sodium and chlorine; water which is a byproduct of hydrogen and oxygen; and rust which is formed when iron reacts with oxygen.
TAGS:
Elements
Compounds
Elements ad Compounds
Elements VS Compounds
Elements and Compounds for Grade 7
Elements and Compounds Chemistry
Elements and Compound Difference
Elements and Compounds Examples
Compound Elements Examples
Elements Chemistry
Compounds Chemistry
Elements Examples
Compounds Examples
Comparing Elements and Compounds
Elements and Compounds Class 7
Classifying Elements and Compounds
Characteristics of Elements and Compounds
Elements Compounds
Комментарии
 0:04:15
0:04:15
 0:02:28
0:02:28
 0:03:49
0:03:49
 0:07:17
0:07:17
 0:05:53
0:05:53
 0:05:53
0:05:53
 0:02:12
0:02:12
 0:05:06
0:05:06
 0:34:47
0:34:47
 0:06:31
0:06:31
 0:13:53
0:13:53
 0:03:41
0:03:41
 0:00:44
0:00:44
 0:04:49
0:04:49
 0:25:13
0:25:13
 0:19:12
0:19:12
 0:45:43
0:45:43
 0:04:21
0:04:21
 0:06:22
0:06:22
 0:02:16
0:02:16
 0:03:57
0:03:57
 0:02:39
0:02:39
 0:04:08
0:04:08
 0:10:36
0:10:36