filmov
tv
Calculating The Relative Atomic Mass of Copper - GCSE Chemistry | kayscience.com

Показать описание
In this video, you will learn this model answer to achieve 100% in any exam question:
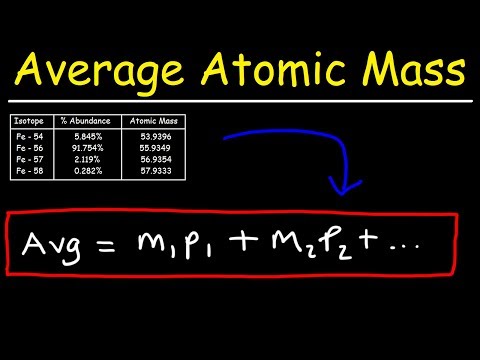
Isotopes are different atoms of the same element, which have the same number of protons and a different number of neutrons. All elements have the same number of protons, however if their atoms have a different number of neutrons, then they are isotopes. Relative atomic mass describes the average mass of all the isotopes. The relative atomic mass of different isotopes can be calculated by:
sum of ((Ar x % abundance)+(Ar x % abundance)+etc...)/100
How To Calculate Relative Atomic Mass | Chemical Calculations | Chemistry | FuseSchool
GCSE Chemistry - Relative Formula Mass #24
Relative Atomic Mass (Ar) - mole concept
Relative Atomic Mass | How to Find Relative Atomic Mass of an Element?
Relative Atomic Mass | Properties of Matter | Chemistry | FuseSchool
GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2
Tutorial Exercises to Calculate Relative Atomic Mass
Calculating Relative Atomic Mass from Mass Spectra
Can we design transistors one atom at a time?
2.1 Calculating Relative Atomic Mass (SL)
Measuring Atomic Mass | Atoms and Molecules | Don't Memorise
How To Find Relative Atomic Mass Of Isotopes With Ratios Given for 2023 JAMB Chemistry
How To Calculate The Average Atomic Mass
2023 JAMB Class on Relative atomic mass Calculations
Matric part 1 Chemistry, Relative Atomic Mass Chemistry - Ch 1 - 9th Class Chemistry
GCSE Chemistry Revision - Calculating Relative Atomic Mass
Calculating The Relative Atomic Mass of Copper - GCSE Chemistry | kayscience.com
How to Calculate Atomic Mass Practice Problems
Relative Formula Mass - mole concept
How To Find The Percent Abundance of Each Isotope - Chemistry
Atomic Mass: How to Calculate Isotope Abundance
2.1 Calculating relative atomic mass (SL)
Atomic Weight: The Convenient Mass of Atoms
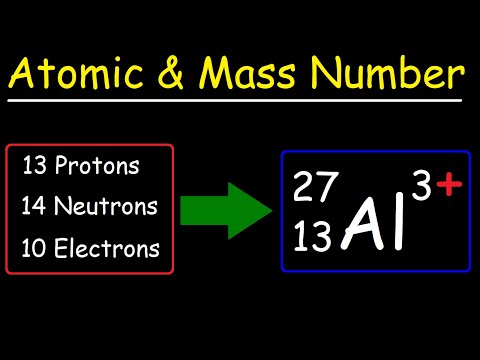
Atomic Number, Mass Number, and Net Electric Charge
Комментарии
 0:03:48
0:03:48
 0:03:59
0:03:59
 0:05:32
0:05:32
 0:07:57
0:07:57
 0:04:24
0:04:24
 0:07:01
0:07:01
 0:05:58
0:05:58
 0:06:18
0:06:18
 0:59:40
0:59:40
 0:04:24
0:04:24
 0:04:16
0:04:16
 0:05:17
0:05:17
 0:07:19
0:07:19
 0:17:14
0:17:14
 0:10:02
0:10:02
 0:01:00
0:01:00
 0:03:39
0:03:39
 0:06:11
0:06:11
 0:05:57
0:05:57
 0:10:18
0:10:18
 0:11:49
0:11:49
 0:03:18
0:03:18
 0:02:58
0:02:58
 0:11:41
0:11:41