filmov
tv
WCLN - Using Indicators to Find Approximate pH
Показать описание
This video shows how the colours of various indicators in a given solution can be used to narrow down the possible pH range of the solution. It contains two example questions that are worked through.
0:07testing samples at the given solution with various indicators
0:11allows one to narrow down the range a possible pH values for the solution
0:15we'll show you how this works using a couple examples
0:19in our first example question were given that three separate samples absolution a
0:25are obtained and each sample is tested with a different indicator
0:29the results are shown on the following table
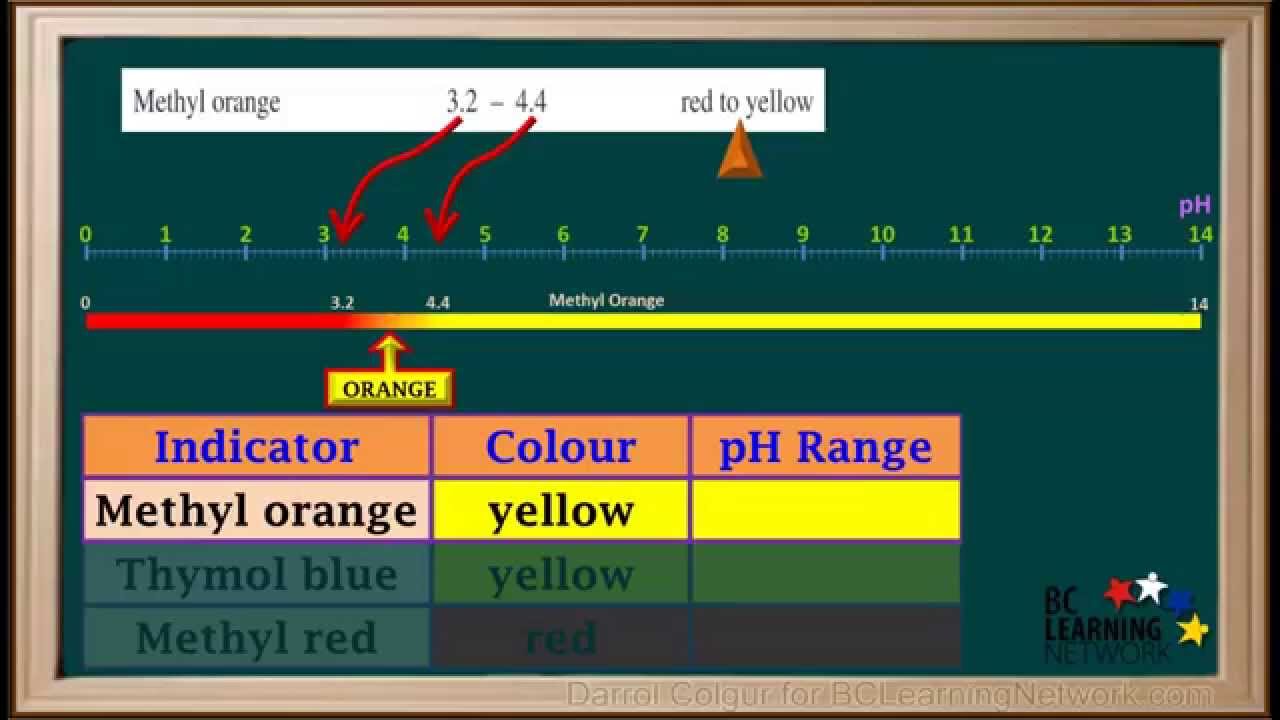
0:33methyl orange yellow Dymo blue jell-o_
0:36and metal red is red
0:39will locate methyl orange metal red and time all blue
0:42on the indicator table it gives us the pH ranges
0:46at the various colors the beach indicator
0:49noticed I'm a blue appears twice on this table
0:54we'll start with methyl orange its line on the indicator table
0:58shown on top here tells us it changes from red to yellow
1:02as the pH increases from 3.2 24.4
1:08so below a pH 3.2 metal oranges rat
1:13between pH 3.2 you and 4.4
1:16it changes from red to orange to yellow as the pH increases
1:22and about the pH up 4.4 it is yellow
1:26the table shown on the bottom here tells us methyl orange
1:30is yellow in solution a
1:32so that houses the pH solution a is greater than or equal to
1:364.4
1:39now look at them all blue its lines on the indicator table
1:42shown on top here tells us that turned yellow
1:46at pH f2.8
1:49and stage lol until the pH increases 28
1:54table at the bottom shows us that time all blue
1:57is yellow into lucien name
2:01so this does is the pH is somewhere between 2.8
2:04in eight
2:06the line from apple red on the indicator table shown on top here
2:10tells us that math already is rad at pH is below
2:144.8
2:17orange between 4.9 8 and six
2:20and yellow and Neph is about six
2:24the table at the bottom shows that metal rad is red
2:27insulation a
2:29so this indicator tells us the pH is less than or equal to you
2:344.8
2:36because the pH is greater than or equal to 4.4
2:40and less than or equal to four point me
2:43it means pH is somewhere between 4.4
2:46and 4.8 inclusive
2:49noticed that the range 4.4 24.8
2:53is well within the range 2.8 28
2:57so the range time are blue gives us
3:00does not help to narrow down the pH range 4.4
3:0424.8 which is our answer
3:08here's another question
3:09skin were given that solution be is yellow
3:12when the indicator alizarin yellow is added and blue
3:15when the indicator them all blue that
3:19and harassed which one did the solutions is to correct identity
3:22for solution B
3:24the indicator table tells us that when alizarin yellow
3:27is yellow the pH is less than or equal to 10 .1
3:33so we'll add this range to our table down here
3:36for solution B
3:38the indicator table also tells us that one time all blues blue
3:42the pH is greater than or equal to 9.6
3:47so well at this range in the table down here
3:50because the pH is greater than or equal to 9.6
3:54and less than or equal to can point one
3:57we can say that the pH is somewhere between 9.6
4:01and 10 .1 inclusive
4:04now will calculate the pH each wanted to give and possible solutions
4:08and see which one fits within the range we came up with
4:12we'll start with 3.2 times tend to the negative
4:15for smaller HCl
4:18because the HCl is a strong acid the concentration at age 30 class
4:22is equal to the concentration of the acid
4:25which is 3.2 times tend to the negative for smaller
4:29pH is the negative log Abidjan a mine concentration
4:34which is the negative log 3.2 times tend to the negative
4:38for it
4:39rounded to one decimal place this comes out to 3.5
4:44so will make a note about here
4:47now will find the pH of the second solution 3.2 times tend to be negative
4:51for smaller
4:52any wage
4:54because any wages strong bays we can say the concentration avoid minus
4:59is equal to the concentration of NaOH
5:02which is 3.2 times tend to be negative for it more
5:06feely ages the negative log the hydroxide ion concentration
0:07testing samples at the given solution with various indicators
0:11allows one to narrow down the range a possible pH values for the solution
0:15we'll show you how this works using a couple examples
0:19in our first example question were given that three separate samples absolution a
0:25are obtained and each sample is tested with a different indicator
0:29the results are shown on the following table
0:33methyl orange yellow Dymo blue jell-o_
0:36and metal red is red
0:39will locate methyl orange metal red and time all blue
0:42on the indicator table it gives us the pH ranges
0:46at the various colors the beach indicator
0:49noticed I'm a blue appears twice on this table
0:54we'll start with methyl orange its line on the indicator table
0:58shown on top here tells us it changes from red to yellow
1:02as the pH increases from 3.2 24.4
1:08so below a pH 3.2 metal oranges rat
1:13between pH 3.2 you and 4.4
1:16it changes from red to orange to yellow as the pH increases
1:22and about the pH up 4.4 it is yellow
1:26the table shown on the bottom here tells us methyl orange
1:30is yellow in solution a
1:32so that houses the pH solution a is greater than or equal to
1:364.4
1:39now look at them all blue its lines on the indicator table
1:42shown on top here tells us that turned yellow
1:46at pH f2.8
1:49and stage lol until the pH increases 28
1:54table at the bottom shows us that time all blue
1:57is yellow into lucien name
2:01so this does is the pH is somewhere between 2.8
2:04in eight
2:06the line from apple red on the indicator table shown on top here
2:10tells us that math already is rad at pH is below
2:144.8
2:17orange between 4.9 8 and six
2:20and yellow and Neph is about six
2:24the table at the bottom shows that metal rad is red
2:27insulation a
2:29so this indicator tells us the pH is less than or equal to you
2:344.8
2:36because the pH is greater than or equal to 4.4
2:40and less than or equal to four point me
2:43it means pH is somewhere between 4.4
2:46and 4.8 inclusive
2:49noticed that the range 4.4 24.8
2:53is well within the range 2.8 28
2:57so the range time are blue gives us
3:00does not help to narrow down the pH range 4.4
3:0424.8 which is our answer
3:08here's another question
3:09skin were given that solution be is yellow
3:12when the indicator alizarin yellow is added and blue
3:15when the indicator them all blue that
3:19and harassed which one did the solutions is to correct identity
3:22for solution B
3:24the indicator table tells us that when alizarin yellow
3:27is yellow the pH is less than or equal to 10 .1
3:33so we'll add this range to our table down here
3:36for solution B
3:38the indicator table also tells us that one time all blues blue
3:42the pH is greater than or equal to 9.6
3:47so well at this range in the table down here
3:50because the pH is greater than or equal to 9.6
3:54and less than or equal to can point one
3:57we can say that the pH is somewhere between 9.6
4:01and 10 .1 inclusive
4:04now will calculate the pH each wanted to give and possible solutions
4:08and see which one fits within the range we came up with
4:12we'll start with 3.2 times tend to the negative
4:15for smaller HCl
4:18because the HCl is a strong acid the concentration at age 30 class
4:22is equal to the concentration of the acid
4:25which is 3.2 times tend to the negative for smaller
4:29pH is the negative log Abidjan a mine concentration
4:34which is the negative log 3.2 times tend to the negative
4:38for it
4:39rounded to one decimal place this comes out to 3.5
4:44so will make a note about here
4:47now will find the pH of the second solution 3.2 times tend to be negative
4:51for smaller
4:52any wage
4:54because any wages strong bays we can say the concentration avoid minus
4:59is equal to the concentration of NaOH
5:02which is 3.2 times tend to be negative for it more
5:06feely ages the negative log the hydroxide ion concentration
 0:07:36
0:07:36
 0:08:38
0:08:38
 0:09:01
0:09:01
 0:02:21
0:02:21
 0:09:26
0:09:26
 0:02:52
0:02:52
 0:03:09
0:03:09
 0:11:37
0:11:37
 0:08:31
0:08:31
 0:08:04
0:08:04
 0:02:36
0:02:36
 0:08:46
0:08:46
 0:04:47
0:04:47
 0:00:33
0:00:33
 0:03:50
0:03:50
 0:06:25
0:06:25
 0:02:14
0:02:14
 0:05:15
0:05:15
 0:00:40
0:00:40
 0:00:52
0:00:52
 0:04:18
0:04:18
 0:04:51
0:04:51
 0:03:15
0:03:15
 0:10:04
0:10:04