filmov
tv
18.3/R3.1.14 Explain how the pH range of an indicator relates to its pKa value [HL IB Chemistry]

Показать описание
If you have chosen your indicator wisely then it will change colour when the acid/base reaction is over (at equivalence point). So find and indicator with pka = pH of your reaction at equivalence point. At this pH the both colours of the indicator will be at equal concentration = colour change.
A satisfying chemical reaction
Olha o que esse garoto fez com ela (@Jullypoca) #shorts #treino #luta #boxing
Jane De Leon nag laplapan live. #Malandi #Wild #Darna
Cirurgia de Fimose
Que? #short
Will Tesla window break my hand?
GET MORE HOURS ON PISO WIFI Without Coins|WIFI HACKS| PAANO MAKA KUHA NG MARAMING ORAS SA PISO WIFI
Cheating in exams😏!?
How to Reset Network Settings on Samsung Galaxy A03s #shorts
magnetic fields lines of solenoid #shorts #class10science #scienceexperiment
Salsa Night in IIT Bombay #shorts #salsa #dance #iit #iitbombay #motivation #trending #viral #jee
Sgt Nantes sempre alerta | ROTA - PMESP | #policia #militar #rota #pm #pmesp #saopaulo #nantes #pm
How To Enable Three-Button Navigation Menu/ Back Button In Android/ Disable Gesture Navigation
DON'T Sell This Bike! 😳
Ek jhatke mein ho jayega The End 💔
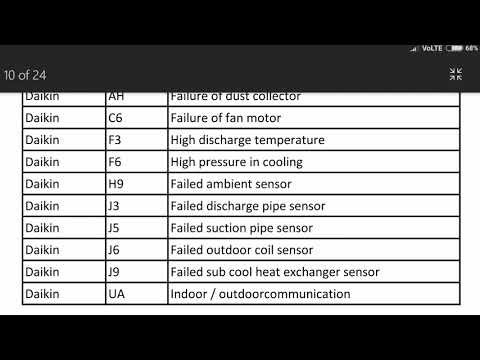
daikin Inverter ac error code list
Fix You're in TalkBack mode | Hold down both volume buttons for 3 seconds to turn off TalkBack
New secret iPad View Trick👍🏻 No split Screen option problem Fix😳😳
Second round NEET result 2023 MP #neet #neet2024 #neet2023 Shahdol govt medical College
REALQUICK EP3: Anong MAGANDA? AMD Ryzen 3, 5, 7 or 9 EXPLAINED 2020 Desktop Processor Buying Guide
Original Rims VS Replica Rims
Snakes on a bike!
GELEDAH GAMING HOUSE MEWAH RRQ‼️ KITA BONGKAR SEMUA RAHASIA SUKSESNYA⁉️
Not So Easy ❌❌NEET JOURNEY (2017 TO 2022) #trending #neet #success #viral #struggle #motivation
Комментарии
 0:00:19
0:00:19
 0:00:26
0:00:26
 0:00:16
0:00:16
 0:00:41
0:00:41
 0:00:18
0:00:18
 0:00:39
0:00:39
 0:00:45
0:00:45
 0:00:32
0:00:32
 0:00:36
0:00:36
 0:00:17
0:00:17
 0:00:14
0:00:14
 0:00:21
0:00:21
 0:01:19
0:01:19
 0:00:24
0:00:24
 0:00:21
0:00:21
 0:00:36
0:00:36
 0:01:34
0:01:34
 0:00:35
0:00:35
 0:00:16
0:00:16
 0:09:41
0:09:41
 0:05:44
0:05:44
 0:00:33
0:00:33
 0:31:21
0:31:21
 0:00:56
0:00:56