filmov
tv
404 - Adiabatic process.

Показать описание
Compression and expansion of an adiabatically isolated gas is accompanied by its heating and cooling.
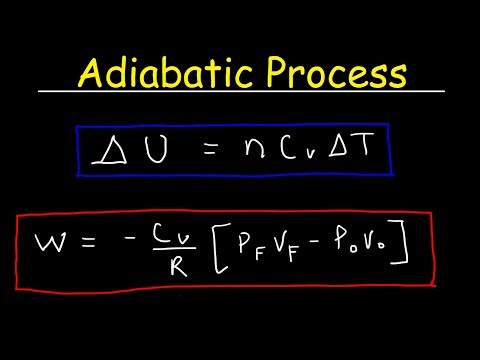
An adiabatic process is one in which no heat is gained or lost by the system. An adiabatic process may be accomplished by thermal insulation or by making rapid changes in volume so that there is no time for heat to be exchanged. The first law of thermodynamics with Q=0 shows that all the change in internal energy is in the form of work done DU = -A. When a gas expands adiabatically it does positive work. The internal energy drops and the temperature drops too. On the contrary, when gas is compressed it does the negative work and the temperature rises. Temperature is the measure of kinetic energy of chaotic motion of the molecules. Higher temperatures correspond to more intense motion of the gas molecules. On a microscopic level it means that when the piston compresses a gas, the speed at which the molecule will be reflected from the piston will be greater than its initial speed. Therefore after reflection from the piston the molecule of gas will receive an additional energy which will be redistributed with time between all molecules of gas due to their mutual collisions.
An adiabatic process is one in which no heat is gained or lost by the system. An adiabatic process may be accomplished by thermal insulation or by making rapid changes in volume so that there is no time for heat to be exchanged. The first law of thermodynamics with Q=0 shows that all the change in internal energy is in the form of work done DU = -A. When a gas expands adiabatically it does positive work. The internal energy drops and the temperature drops too. On the contrary, when gas is compressed it does the negative work and the temperature rises. Temperature is the measure of kinetic energy of chaotic motion of the molecules. Higher temperatures correspond to more intense motion of the gas molecules. On a microscopic level it means that when the piston compresses a gas, the speed at which the molecule will be reflected from the piston will be greater than its initial speed. Therefore after reflection from the piston the molecule of gas will receive an additional energy which will be redistributed with time between all molecules of gas due to their mutual collisions.
Комментарии
 0:01:12
0:01:12
 0:00:38
0:00:38
 0:03:58
0:03:58
 0:00:36
0:00:36
 0:00:10
0:00:10
 0:00:46
0:00:46
 0:03:43
0:03:43
 0:00:52
0:00:52
 0:10:46
0:10:46
 0:00:10
0:00:10
 0:00:23
0:00:23
 0:00:24
0:00:24
 0:00:56
0:00:56
 0:10:38
0:10:38
 0:01:10
0:01:10
 0:02:05
0:02:05
 0:00:13
0:00:13
 0:11:43
0:11:43
 0:00:12
0:00:12
 0:04:31
0:04:31
 0:13:00
0:13:00
 0:02:09
0:02:09
 0:16:00
0:16:00
 0:01:46
0:01:46