filmov
tv
Finding Oxidation Numbers Practice Problems and Answers

Показать описание
To become skilled at finding oxidation numbers you need lots of practice. In this video you’ll be presented with nine practice problems that become increasingly difficult.
First, calculate the oxidation numbers for a compound, then go to the time listed in the video for a full explanation. Think of it as a video worksheet on oxidation numbers.
--- General Rules for Determining Oxidation Numbers ---
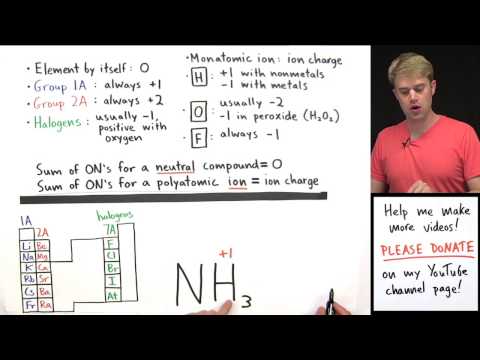
1. In a neutral compound all oxidation numbers must add up to zero.
2. In an ion the all oxidation numbers must add up to the charge on the ion.
3. Free elements have an oxidation number of zero (e.g. Na, Fe, H2, O2, S8).
4. Fluorine (F) = -1
5. Group 1 = +1 Group 2 = +2
6.Hydrogen with Non-Metals = +1 Hydrogen with Metals (or Boron) = -1
7. Oxygen = -2 (except with Fluorine or Peroxides)
8. Group 17(7A) = -1 Group 16 (6A) = -2 Group 15 (5A) = -3
First, calculate the oxidation numbers for a compound, then go to the time listed in the video for a full explanation. Think of it as a video worksheet on oxidation numbers.
--- General Rules for Determining Oxidation Numbers ---
1. In a neutral compound all oxidation numbers must add up to zero.
2. In an ion the all oxidation numbers must add up to the charge on the ion.
3. Free elements have an oxidation number of zero (e.g. Na, Fe, H2, O2, S8).
4. Fluorine (F) = -1
5. Group 1 = +1 Group 2 = +2
6.Hydrogen with Non-Metals = +1 Hydrogen with Metals (or Boron) = -1
7. Oxygen = -2 (except with Fluorine or Peroxides)
8. Group 17(7A) = -1 Group 16 (6A) = -2 Group 15 (5A) = -3
How to Calculate Oxidation Number Practice Problems
Finding Oxidation Numbers Practice Problems and Answers
How To Calculate Oxidation Numbers - Basic Introduction
How to Find Oxidation Numbers (Rules and Examples)
How to Calculate Oxidation Numbers Introduction
Oxidation Number Practice - Extra practice problems assigning oxidation numbers
Practice determining oxidation states | Chemistry | Khan Academy
IIT/JEE Chemistry Practice #5: Oxidation Numbers
How to Determine the Oxidation Number from Lewis Structure Examples & Practice Problems
Oxidation and Reduction (Redox) Reactions Step-by-Step Example
How to find oxidation states: practice problems - Real Chemistry
Assigning Oxidation Numbers - Chemistry Tutorial
Determine Oxidation Numbers Practice
Oxidation and Reduction Reactions - Basic Introduction
Oxidation Numbers Practice Problems
How To Calculate Oxidation Number or Oxidation State? Easy Trick
Oxidation Number Simplified: Oxidation Number Rules with Many Examples and Practice Problems
Calculating Oxidation Number
Oxidation Number Examples
Oxidation-Reduction Reactions
The Oxidation Reduction Question that Tricks Everyone!
Rules for Oxidation Numbers
#isequaltoklasses | OXIDATION NUMBERS | SOLVED EXAMPLES |
WCLN - Finding Oxidation Numbers Example 2 - Chemistry
Комментарии
 0:15:25
0:15:25
 0:10:01
0:10:01
 0:31:15
0:31:15
 0:07:00
0:07:00
 0:13:26
0:13:26
 0:04:50
0:04:50
 0:04:27
0:04:27
 0:07:00
0:07:00
 0:05:56
0:05:56
 0:03:56
0:03:56
 0:07:08
0:07:08
 0:06:00
0:06:00
 0:13:43
0:13:43
 0:16:05
0:16:05
 0:05:41
0:05:41
 0:13:13
0:13:13
 0:09:23
0:09:23
 0:11:06
0:11:06
 0:06:22
0:06:22
 0:03:52
0:03:52
 0:06:19
0:06:19
 0:04:50
0:04:50
 0:08:31
0:08:31
 0:03:04
0:03:04