filmov
tv
Calculating with Moles & Molar Mass in Chemistry

Показать описание
This tutorial delves into the foundational concept of calculating with moles, a crucial skill for students, educators, and professionals in chemistry. The mole is a fundamental unit in chemistry that provides a bridge between the microscopic world of atoms, molecules, and ions and the macroscopic world we can measure. Understanding how to perform calculations with moles is essential for mastering stoichiometry, chemical reactions, and solution concentration calculations. This video aims to simplify mole calculations, making them accessible and comprehensible for all viewers.
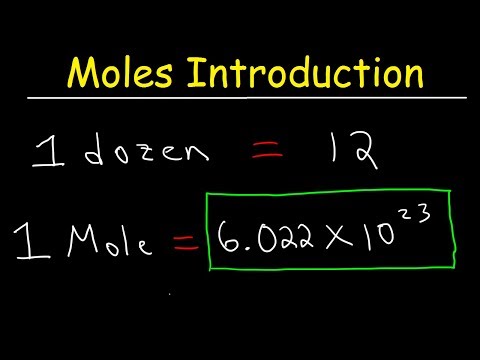
The video begins with an explanation of the mole concept, including Avogadro's number and its significance in relating mass to the number of particles. It then progresses to cover various types of mole calculations, such as converting between moles and mass, moles and volume of gases at standard temperature and pressure (STP), and moles and solutions' molarity.
Through clear, step-by-step examples, viewers will learn the methods and formulas necessary to perform these conversions effectively. The tutorial also addresses common misconceptions and pitfalls encountered in mole calculations, providing tips for avoiding errors and ensuring accuracy.
By the end of this tutorial, viewers will have a solid grasp of calculating with moles, enhancing their ability to solve a wide range of chemical problems. Join us to master this essential aspect of chemical quantification.
The video begins with an explanation of the mole concept, including Avogadro's number and its significance in relating mass to the number of particles. It then progresses to cover various types of mole calculations, such as converting between moles and mass, moles and volume of gases at standard temperature and pressure (STP), and moles and solutions' molarity.
Through clear, step-by-step examples, viewers will learn the methods and formulas necessary to perform these conversions effectively. The tutorial also addresses common misconceptions and pitfalls encountered in mole calculations, providing tips for avoiding errors and ensuring accuracy.
By the end of this tutorial, viewers will have a solid grasp of calculating with moles, enhancing their ability to solve a wide range of chemical problems. Join us to master this essential aspect of chemical quantification.
Комментарии
 0:04:29
0:04:29
 0:07:25
0:07:25
 0:05:49
0:05:49
 0:11:46
0:11:46
 0:11:20
0:11:20
 0:35:31
0:35:31
 0:05:16
0:05:16
 0:06:04
0:06:04
 0:00:28
0:00:28
 0:10:47
0:10:47
 0:05:02
0:05:02
 0:12:11
0:12:11
 0:24:42
0:24:42
 0:25:16
0:25:16
 0:19:58
0:19:58
 0:07:23
0:07:23
 0:05:29
0:05:29
 0:01:25
0:01:25
 0:06:06
0:06:06
 0:05:57
0:05:57
 0:04:56
0:04:56
 0:03:14
0:03:14
 0:13:11
0:13:11
 0:11:03
0:11:03