filmov
tv
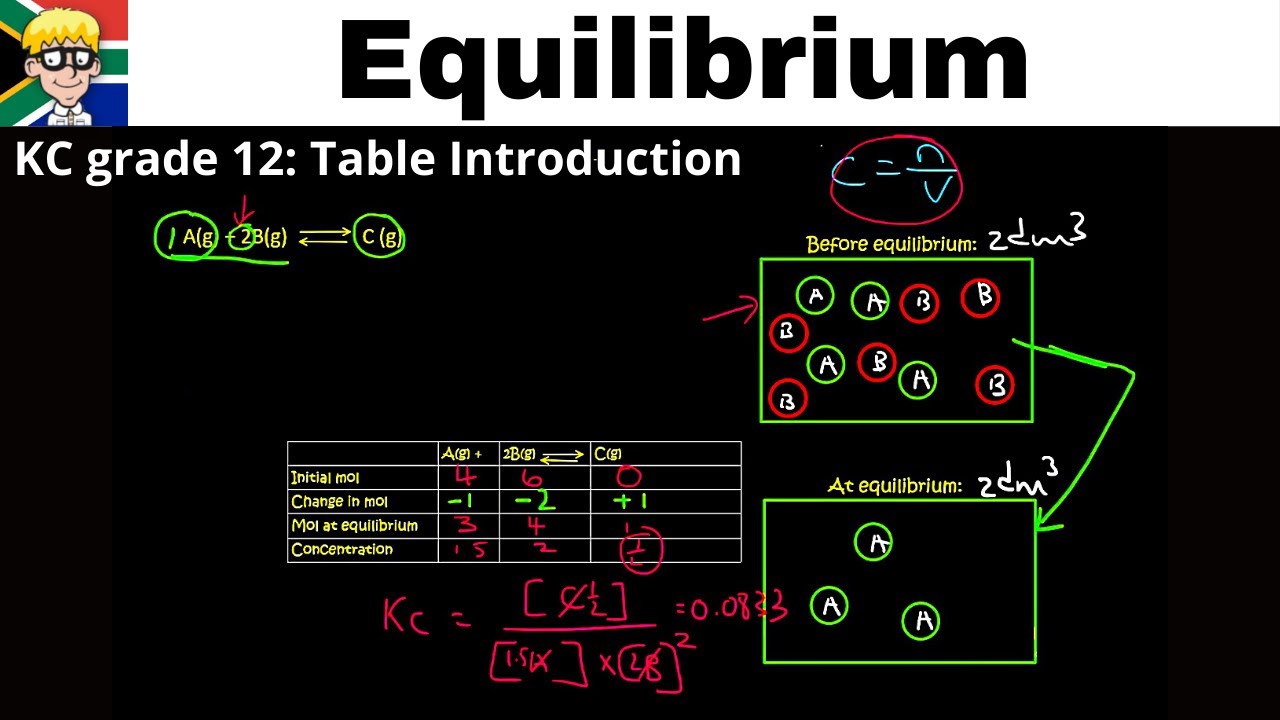
KC grade 12: Table Introduction
Показать описание
In this lesson we learn how to use a KC table
KC grade 12: Table Introduction
KC grade 12: Introduction
KC grade 12: Table Practice
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
Equilibrium Constant Grade 12: Exam
Equilibrium Constant Grade 12: Practice
Equilibrium Constant Grade 12: Exam
Chemical Equilibria and Reaction Quotients
Equilibrium Graphs grade 12: Introduction
Le Chatelier's Principle grade 12: Introduction
Equilibrium: Crash Course Chemistry #28
Kc Calculations and RICE Tables
Equilibrium constant (Kc) introduction
How to calculate the Equilibrium constant (Kc) Grade 12 - Explained in the simplest way
Equilibrium Made Easy: How to Solve Chemical Equilibrium Problems
KC Equilibrium Constant Grade 12 Physical Sciences P2 Free Saturday School Class
Chemistry_Calculating the Equilibrium constant
Boyle’s Law
Exam Equilibrium Grade 12
Grade 12 Physical Science | 32. Chemical Equilibrium and Equilibrium Constant by 123tutors
Le Chatelier's Principle
Trigonometry Class 10 | Trigonometry Identities| Trigonometry Formulas #fun #shorts #youtubeshorts
Using RICE to calculate equilibrium concentrations
Grade 12 Chemistry - Equilibrium Constant 1
Комментарии
 0:08:02
0:08:02
 0:02:12
0:02:12
 0:04:01
0:04:01
 0:53:22
0:53:22
 0:06:48
0:06:48
 0:10:30
0:10:30
 0:06:33
0:06:33
 0:06:48
0:06:48
 0:03:26
0:03:26
 0:02:41
0:02:41
 0:10:56
0:10:56
 0:14:41
0:14:41
 0:08:58
0:08:58
 0:11:30
0:11:30
 0:12:43
0:12:43
 0:16:12
0:16:12
 0:51:35
0:51:35
 0:00:15
0:00:15
 0:07:48
0:07:48
 0:24:28
0:24:28
 0:26:40
0:26:40
 0:00:14
0:00:14
 0:10:13
0:10:13
 0:31:50
0:31:50