filmov
tv
R2.2.13 Calculating activation energy (HL)

Показать описание
This video covers how to calculate activation energy.
Determining activation energy, sample data IB Chemistry HL 16.2
Week 6 - 2. Measuring Activation energy from an Arrhenius plot
R2.2.6 Reaction mechanism, order of reaction and rate-determining step [HL IB Chemistry]
R2.2.6 Intermediates and catalysts (HL)
Kinetics: Initial Rates and Integrated Rate Laws
How to Determine Activation Energy Using Nonlinear Regression
Apparent Activation Energy
Determining Activation Energy using Arrhenius Equation
Calculate Activation Energy Using Arrhenius' Equation 006
Arrhenius Equation | CHEM 1302 @ Western
IB Chemistry Topic 6 Kinetics 16.2 Activation energy
Arrhenius plot
16.2 Arrhenius Equation k1,k2,T1,T2 [HL IB Chemistry]
AQA Arrhenius Plots 1
Determining a rate law from a proposed reaction mechanism Sp 13 A14
16.2 The Arrhenius equation (HL)
Chem162 Activation Energy and Arrhenius Equation (13.4)
Arrhenius Plots
The Arrhenius Equation
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Step - Chemical Kinetics
R2.2.8 Molecularity (HL)
The Logarithmic Arrhenius Equation. Y13 A-level Chemistry OCR AQA: Reaction Rates Kinetics
14.5 The Arrhenius Equation
Reaction Mechanisms (IB Chemistry R2.2)
Комментарии
 0:04:41
0:04:41
 0:05:39
0:05:39
 0:09:02
0:09:02
 0:05:27
0:05:27
 0:09:10
0:09:10
 0:04:24
0:04:24
 0:08:48
0:08:48
 0:06:41
0:06:41
 0:09:48
0:09:48
 0:02:30
0:02:30
 0:03:31
0:03:31
 0:04:22
0:04:22
 0:07:05
0:07:05
 0:05:27
0:05:27
 0:04:21
0:04:21
 0:03:11
0:03:11
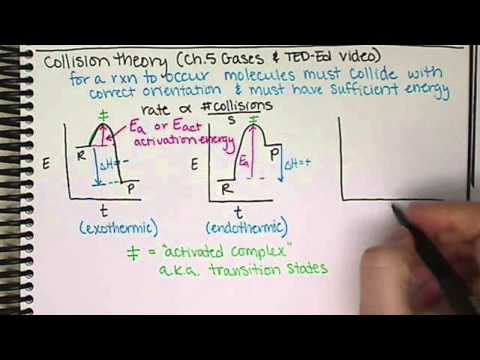 0:13:12
0:13:12
 0:10:50
0:10:50
 0:08:24
0:08:24
 0:18:48
0:18:48
 0:02:47
0:02:47
 0:05:44
0:05:44
 0:05:54
0:05:54
 0:18:11
0:18:11