filmov
tv
16.2 Arrhenius Equation k1,k2,T1,T2 [HL IB Chemistry]

Показать описание
Changing temps for a reaction will change the rate constant. The equation allows this to be calculated, if you know the 3 of 4 variables (the different rate constants at different temps).
The Arrhenius Equation
Collision Theory - Arrhenius Equation & Activation Energy - Chemical Kinetics
Forms of the Arrhenius equation | Kinetics | Chemistry | Khan Academy
Using the Arrhenius equation | Kinetics | Chemistry | Khan Academy
arrhenius equation example 2
The Arrhenius Equation
Quick video: Application of Arrhenius equation
The Arrhenius equation. Applications
3 / 4 - Lecture 17 - Solving for activation energy with rate constants at two different temperatures
arrhenius equation example
Energy of Activation From Arrhenius Equation Solved Problem
4 / 4 - Lecture 17 - Example problem of solving for Activation Energy
Arrhenius Equation Part1
Temperature Dependence Of Reaction Rate|Arrhenius Equation|Activation Energy|Gardin Equation
14.5 The Arrhenius Equation
Arrhenius Equation
Arrhenius Equation - Chemical Kinetics #14
Arrhenius Equation
03 11 arrhenius equation sample problem
Arrhenius Equation
16.8 Sample Problem
Calculate the activation energy of a reaction
Chemical Kinetics -IV- Role of pressure & temperature on reaction rate| Arrhenius Equation
Kinetics Collision Theory Arrhenius Eqn
Комментарии
 0:05:41
0:05:41
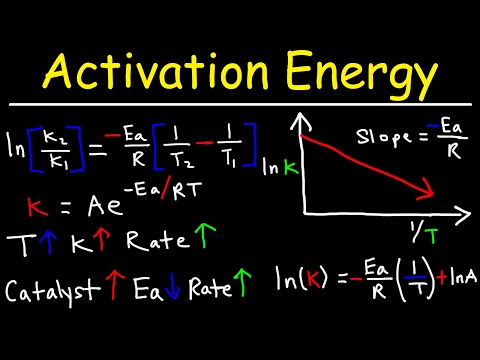 0:31:50
0:31:50
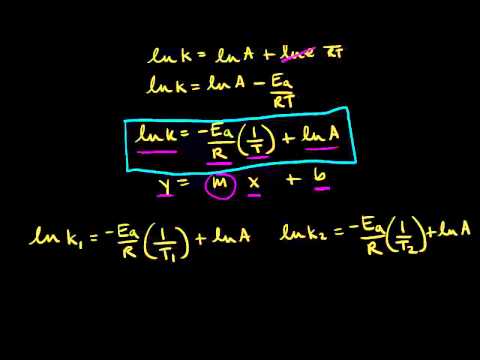 0:06:41
0:06:41
 0:11:06
0:11:06
 0:04:44
0:04:44
 0:07:09
0:07:09
 0:09:29
0:09:29
 0:06:31
0:06:31
 0:07:10
0:07:10
 0:06:09
0:06:09
 0:07:00
0:07:00
 0:07:35
0:07:35
 0:50:34
0:50:34
 0:34:49
0:34:49
 0:05:54
0:05:54
 0:19:51
0:19:51
 0:08:29
0:08:29
 0:05:16
0:05:16
 0:10:27
0:10:27
 0:11:49
0:11:49
 0:07:38
0:07:38
 0:09:33
0:09:33
 0:16:42
0:16:42
 0:07:33
0:07:33