filmov
tv
What is percentage purity? How to calculate percent purity? - Dr K

Показать описание
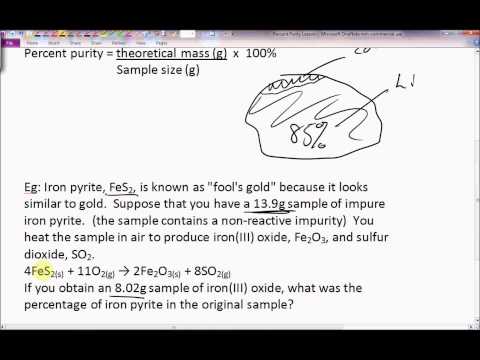
In this video, we are going to address these questions: What is percentage purity? How to calculate percent purity? Percentage purity or percent purity tells us how pure a sample is. Percent purity can be calculated by taking the mass of a pure sample and divide it by the mass of the impure sample times 100. Sometimes, the mass of the impure sample is referred to as the total mass of a compound.
We will use a percent purity example in this video to demonstrate how to calculate percent purity:
4.98 g of CO2 is produced when diluted HCl is added to 11.67 g of contaminated CaCO3. Calculate the % purity of CaCO3.
In the question, we are given the mass of impure sample. However, in order to proceed, we need to first figure out how to calculate mass of pure sample. This is a simple stoichiometry problem where we convert from grams of CO2 to grams of CaCO3. Watch the video to see how this calculation is performed and ultimately how it is applied in the percent purity formula.
🕑 Here's when you'll find:
0:00 What is percentage purity?
0:17 How to calculate percent purity?
0:27 Percent purity example
1:11 How to calculate mass of pure sample?
3:28 Steps required to calculate percent purity
Related videos:
* Closed captioning is available in English *
👍Subscribe for more Chemistry videos 👍
PLAYLISTS:
💗YOU MAY ALSO LIKE:
Facebook: @ChemSimplified
🎵 Music: Heavenly by Aakash Gandhi
We will use a percent purity example in this video to demonstrate how to calculate percent purity:
4.98 g of CO2 is produced when diluted HCl is added to 11.67 g of contaminated CaCO3. Calculate the % purity of CaCO3.
In the question, we are given the mass of impure sample. However, in order to proceed, we need to first figure out how to calculate mass of pure sample. This is a simple stoichiometry problem where we convert from grams of CO2 to grams of CaCO3. Watch the video to see how this calculation is performed and ultimately how it is applied in the percent purity formula.
🕑 Here's when you'll find:
0:00 What is percentage purity?
0:17 How to calculate percent purity?
0:27 Percent purity example
1:11 How to calculate mass of pure sample?
3:28 Steps required to calculate percent purity
Related videos:
* Closed captioning is available in English *
👍Subscribe for more Chemistry videos 👍
PLAYLISTS:
💗YOU MAY ALSO LIKE:
Facebook: @ChemSimplified
🎵 Music: Heavenly by Aakash Gandhi
Комментарии
 0:04:24
0:04:24
 0:04:41
0:04:41
 0:05:34
0:05:34
 0:05:03
0:05:03
 0:14:08
0:14:08
 0:16:58
0:16:58
 0:13:13
0:13:13
 0:05:03
0:05:03
 0:00:26
0:00:26
 0:13:23
0:13:23
 0:01:33
0:01:33
 0:04:54
0:04:54
 0:08:04
0:08:04
 0:03:08
0:03:08
 0:00:16
0:00:16
 0:06:24
0:06:24
 0:05:45
0:05:45
 0:04:48
0:04:48
 0:03:10
0:03:10
 0:01:42
0:01:42
 0:05:13
0:05:13
 0:12:54
0:12:54
 0:10:46
0:10:46
 0:09:20
0:09:20