filmov
tv
Resonance in Chemistry Explained in Simple Words with Examples

Показать описание
Resonance is a way of describing delocalized electrons within certain molecules where a single Lewis formula cannot express the bonding. To understand resonance in chemistry, you need to first understand covalent bonds, sigma and pi bonding and Lewis structures.
There are three main types of bonds, dependent on the number of electrons shared between atoms. If two atoms within a molecule share a single pair of electrons, a single bond is formed between the atoms. Similarly, double and triple bonds are formed when two atoms share two and three pairs of electrons, respectively.
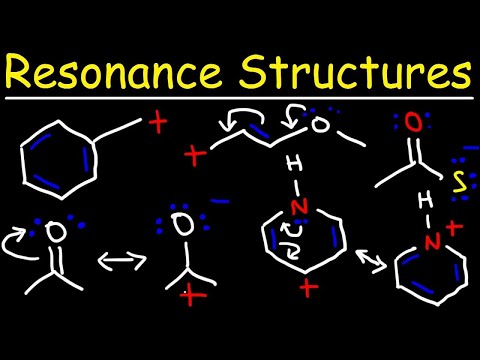
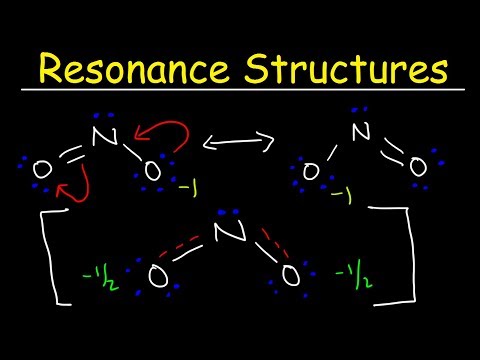
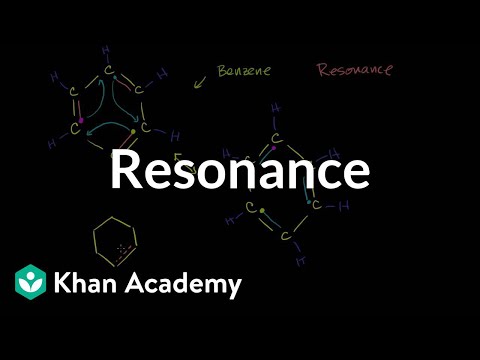
Covalent bonds also come in two types: sigma bonds and pi bonds. A Lewis Structure is a tool to represent valence shell electrons in a molecule. A Lewis structure shows the arrangement of electrons around individual atoms in the molecule. Electrons are shown as dots, while bonding electrons are denoted as a line between the two atoms. electrons in sigma bonds tend to be in specific locations, which is why those electrons are said to be localized, whereas pi bonds have delocalized electrons; these are found above and below the atoms and are spread across several atoms. The idea of resonance helps us understand and describe molecules that a single Lewis structure cannot represent. The various resonance structures show different possibilities of the electrons’ positions in the molecule.
Table of Content
Introduction: 0:00
Resonance in Chemistry: 0:34
Covalent Bonds and Electron Sharing: 1:11
Types of Covalent Bonds: 1:39
Sigma and Pi Bonds: 2:00
Lewis Structures: 3:14
Resonance and Delocalized Electrons: 3:33
Application of Resonance - Benzene: 4:03
Conclusion: 4:57
#ChemicalResonance #MolecularStructure #ElectronDynamics
References:
SUBSCRIBE to get more such science videos!
Follow us on Twitter!
Follow us on Facebook!
Follow us on Instagram!
Follow us on LinkedIn!
Follow our Website!
There are three main types of bonds, dependent on the number of electrons shared between atoms. If two atoms within a molecule share a single pair of electrons, a single bond is formed between the atoms. Similarly, double and triple bonds are formed when two atoms share two and three pairs of electrons, respectively.
Covalent bonds also come in two types: sigma bonds and pi bonds. A Lewis Structure is a tool to represent valence shell electrons in a molecule. A Lewis structure shows the arrangement of electrons around individual atoms in the molecule. Electrons are shown as dots, while bonding electrons are denoted as a line between the two atoms. electrons in sigma bonds tend to be in specific locations, which is why those electrons are said to be localized, whereas pi bonds have delocalized electrons; these are found above and below the atoms and are spread across several atoms. The idea of resonance helps us understand and describe molecules that a single Lewis structure cannot represent. The various resonance structures show different possibilities of the electrons’ positions in the molecule.
Table of Content
Introduction: 0:00
Resonance in Chemistry: 0:34
Covalent Bonds and Electron Sharing: 1:11
Types of Covalent Bonds: 1:39
Sigma and Pi Bonds: 2:00
Lewis Structures: 3:14
Resonance and Delocalized Electrons: 3:33
Application of Resonance - Benzene: 4:03
Conclusion: 4:57
#ChemicalResonance #MolecularStructure #ElectronDynamics
References:
SUBSCRIBE to get more such science videos!
Follow us on Twitter!
Follow us on Facebook!
Follow us on Instagram!
Follow us on LinkedIn!
Follow our Website!
Комментарии
 0:12:32
0:12:32
 0:05:17
0:05:17
 0:13:14
0:13:14
 0:08:25
0:08:25
 0:10:31
0:10:31
 0:11:46
0:11:46
 0:04:09
0:04:09
 0:20:27
0:20:27
 1:39:55
1:39:55
 0:11:51
0:11:51
 0:00:59
0:00:59
 0:04:01
0:04:01
 0:41:58
0:41:58
 0:18:03
0:18:03
 0:12:00
0:12:00
 0:00:26
0:00:26
 0:29:30
0:29:30
 0:05:25
0:05:25
 0:03:37
0:03:37
 0:27:48
0:27:48
 0:01:01
0:01:01
 0:09:58
0:09:58
 0:12:13
0:12:13
 0:00:43
0:00:43