filmov
tv
Application of Keq & Reaction Quotient, L-7 | By Amit Sir | JEE - NEET -CET | ASQUARE Academy

Показать описание
Welcome to this in-depth session on "Application of Equilibrium Constant, Reaction Quotient & Degree of Dissociation" presented by Amit Sir from ASQUARE Academy. This lecture is part of the Chemical Equilibrium series, designed specifically for JEE, NEET, and CET aspirants, especially students of Class 11th. In this lesson, Amit Sir provides a detailed explanation of key concepts such as the Equilibrium Constant (Kc & Kp), Reaction Quotient (Q), and Degree of Dissociation and their applications in solving chemical equilibrium problems.
Key Concepts Covered:
🔹 Equilibrium Constant (Kc & Kp) Applications:
Learn how to apply the equilibrium constant to determine the extent of a reaction and predict the direction in which the reaction will proceed.
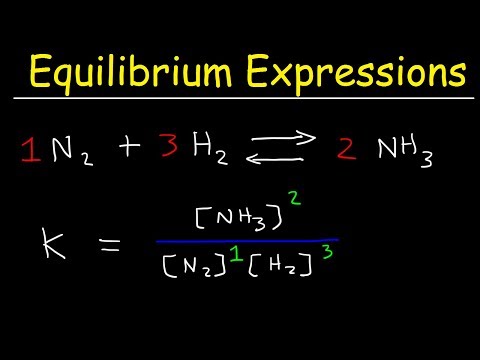
Understand the difference between Kc (concentration) and Kp (partial pressure) and how they are used in different types of reactions (homogeneous and heterogeneous equilibria).
Study how the magnitude of K gives insight into whether products or reactants are favored at equilibrium.
🔹 Reaction Quotient (Q) and its Importance:
The Reaction Quotient (Q) helps in determining the direction of a reaction at any given point in time before equilibrium is reached.
Amit Sir explains how to compare Q with K to predict whether the reaction will move toward the forward or backward direction.
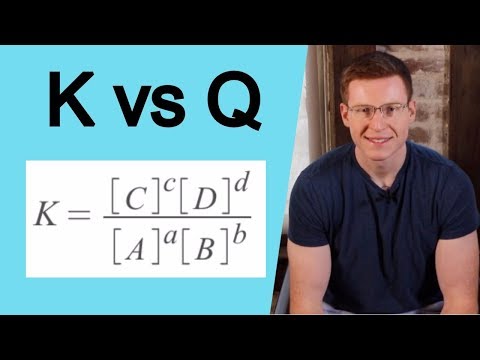
Q smaller K: Reaction proceeds in the forward direction to reach equilibrium.
Q grater K: Reaction proceeds in the backward direction to reach equilibrium.
Q = K: The reaction is at equilibrium.
🔹 Degree of Dissociation:
Explore the concept of degree of dissociation (α) and how it is related to the equilibrium constant, especially in reactions involving weak acids, weak bases, and ionic dissociation.
Learn to calculate the degree of dissociation for weak electrolytes and understand how this affects the concentration of ions in solution.
🔹 Le Chatelier’s Principle:
Application of Le Chatelier’s Principle to predict the effect of changes in concentration, pressure, temperature, and catalysts on the position of equilibrium.
Understand how equilibrium shifts when external conditions are altered.
🔹 Relationship between Kc and Kp:
Learn the relationship between Kc and Kp and how they are interconverted using the formula:
Kp=Kc(RT) Δn
where Δn is the change in the number of moles of gas.
🔹 Solved Examples & Numerical Problems:
Amit Sir solves numerical problems to illustrate the application of Kc, Kp, and Q in determining the equilibrium position, calculating concentrations of reactants/products, and finding the degree of dissociation.
These examples help in better understanding how to approach questions related to equilibrium in competitive exams like JEE, NEET, and CET.
Why Watch This Video?
For Class 11th CBSE & HSC Board Students: This video strengthens your foundation in chemical equilibrium, which is critical for your board exams.
For JEE, NEET & CET Aspirants: Mastering equilibrium concepts is crucial for solving advanced problems in competitive exams.
Stay Connected with ASQUARE Academy:
🔔 Subscribe: Stay updated with more lectures and revision shorts from Amit Sir.
👍 Like & Share: If this video helps you, don’t forget to like and share it with friends.
💬 Comments: Drop your queries and feedback in the comments section for further clarifications.
Follow Us for More:
Facebook: ASQUARE Academy
Instagram: @asquarepune
X (formerly Twitter): @asquarepune
#EquilibriumConstant #ReactionQuotient #DegreeOfDissociation #ChemicalEquilibrium #KcKp #LeChateliersPrinciple #AmitSir #JEE2025 #NEET2025 #CET2025 #ASQUAREAcademy #BestJEEAcademy #BestNEETAcademy #PhysicalChemistry #CompetitiveExamPrep #Class11Chemistry #CBSEBoard #HSCBoard #ChemistryConcepts #StudyWithASQUARE #ExamPreparation #ChemicalReactions #ChemistryShorts #OnlineLearning #JEEPrep #NEETPrep #CETPrep #PuneCoaching #QuickLearning #ScienceEducation #ChemicalEquilibriumLecture #ASQUAREClassroom #PhysicsJEE #BiologyNEET #CETEngineering #NEETMockExams #BoardPreparation #ASQUAREDigital #ChemistryShorts #NEETTips
#MolecularChemistry#PhysicsNEET #StudyWithASQUARE #JEEQuickRevision #NEET2025Prep #CETStudyTips #TopCETClasses #SciencePrep #MedicalEntrancePrep #JEE2025MockTests #BestCETCoaching #StudyMaterials #ExamStrategy #TopPuneClasses #CETConcepts #NEETExam2025 #IITJEE2025 #Class11Physics #ChemistryRevision #ASQUAREPune #CompetitiveExamsPrep #NEETPhysics2025 #JEE2025Revision #NEET2025Concepts #CET2025Tips #StudyWithAmitSir #NEETStudyMaterial #JEEPhysicsProblems #ASQUAREChemistry #JEEChemistry2025 #PuneCoachingClasses #CETExamPreparation #BestScienceAcademy #NEETSuccessTips #TopEngineeringCoaching #ASQUAREBiology #ASQUARECompetitiveExams #Class12NEETChemistry #JEEPhysicsConcepts #MHTCETEngineering #Class11Chemistry #ExamPreparationTips #NEET2025Revision #CETCoaching2025
Key Concepts Covered:
🔹 Equilibrium Constant (Kc & Kp) Applications:
Learn how to apply the equilibrium constant to determine the extent of a reaction and predict the direction in which the reaction will proceed.
Understand the difference between Kc (concentration) and Kp (partial pressure) and how they are used in different types of reactions (homogeneous and heterogeneous equilibria).
Study how the magnitude of K gives insight into whether products or reactants are favored at equilibrium.
🔹 Reaction Quotient (Q) and its Importance:
The Reaction Quotient (Q) helps in determining the direction of a reaction at any given point in time before equilibrium is reached.
Amit Sir explains how to compare Q with K to predict whether the reaction will move toward the forward or backward direction.
Q smaller K: Reaction proceeds in the forward direction to reach equilibrium.
Q grater K: Reaction proceeds in the backward direction to reach equilibrium.
Q = K: The reaction is at equilibrium.
🔹 Degree of Dissociation:
Explore the concept of degree of dissociation (α) and how it is related to the equilibrium constant, especially in reactions involving weak acids, weak bases, and ionic dissociation.
Learn to calculate the degree of dissociation for weak electrolytes and understand how this affects the concentration of ions in solution.
🔹 Le Chatelier’s Principle:
Application of Le Chatelier’s Principle to predict the effect of changes in concentration, pressure, temperature, and catalysts on the position of equilibrium.
Understand how equilibrium shifts when external conditions are altered.
🔹 Relationship between Kc and Kp:
Learn the relationship between Kc and Kp and how they are interconverted using the formula:
Kp=Kc(RT) Δn
where Δn is the change in the number of moles of gas.
🔹 Solved Examples & Numerical Problems:
Amit Sir solves numerical problems to illustrate the application of Kc, Kp, and Q in determining the equilibrium position, calculating concentrations of reactants/products, and finding the degree of dissociation.
These examples help in better understanding how to approach questions related to equilibrium in competitive exams like JEE, NEET, and CET.
Why Watch This Video?
For Class 11th CBSE & HSC Board Students: This video strengthens your foundation in chemical equilibrium, which is critical for your board exams.
For JEE, NEET & CET Aspirants: Mastering equilibrium concepts is crucial for solving advanced problems in competitive exams.
Stay Connected with ASQUARE Academy:
🔔 Subscribe: Stay updated with more lectures and revision shorts from Amit Sir.
👍 Like & Share: If this video helps you, don’t forget to like and share it with friends.
💬 Comments: Drop your queries and feedback in the comments section for further clarifications.
Follow Us for More:
Facebook: ASQUARE Academy
Instagram: @asquarepune
X (formerly Twitter): @asquarepune
#EquilibriumConstant #ReactionQuotient #DegreeOfDissociation #ChemicalEquilibrium #KcKp #LeChateliersPrinciple #AmitSir #JEE2025 #NEET2025 #CET2025 #ASQUAREAcademy #BestJEEAcademy #BestNEETAcademy #PhysicalChemistry #CompetitiveExamPrep #Class11Chemistry #CBSEBoard #HSCBoard #ChemistryConcepts #StudyWithASQUARE #ExamPreparation #ChemicalReactions #ChemistryShorts #OnlineLearning #JEEPrep #NEETPrep #CETPrep #PuneCoaching #QuickLearning #ScienceEducation #ChemicalEquilibriumLecture #ASQUAREClassroom #PhysicsJEE #BiologyNEET #CETEngineering #NEETMockExams #BoardPreparation #ASQUAREDigital #ChemistryShorts #NEETTips
#MolecularChemistry#PhysicsNEET #StudyWithASQUARE #JEEQuickRevision #NEET2025Prep #CETStudyTips #TopCETClasses #SciencePrep #MedicalEntrancePrep #JEE2025MockTests #BestCETCoaching #StudyMaterials #ExamStrategy #TopPuneClasses #CETConcepts #NEETExam2025 #IITJEE2025 #Class11Physics #ChemistryRevision #ASQUAREPune #CompetitiveExamsPrep #NEETPhysics2025 #JEE2025Revision #NEET2025Concepts #CET2025Tips #StudyWithAmitSir #NEETStudyMaterial #JEEPhysicsProblems #ASQUAREChemistry #JEEChemistry2025 #PuneCoachingClasses #CETExamPreparation #BestScienceAcademy #NEETSuccessTips #TopEngineeringCoaching #ASQUAREBiology #ASQUARECompetitiveExams #Class12NEETChemistry #JEEPhysicsConcepts #MHTCETEngineering #Class11Chemistry #ExamPreparationTips #NEET2025Revision #CETCoaching2025
 0:06:48
0:06:48
 0:09:06
0:09:06
 0:04:53
0:04:53
 0:06:15
0:06:15
 0:06:27
0:06:27
 0:04:47
0:04:47
 0:03:00
0:03:00
 0:07:48
0:07:48
 0:26:40
0:26:40
 0:03:54
0:03:54
 0:05:24
0:05:24
 0:07:54
0:07:54
 0:08:41
0:08:41
 0:05:25
0:05:25
 0:07:31
0:07:31
 0:09:27
0:09:27
 0:06:15
0:06:15
 0:10:13
0:10:13
 0:12:30
0:12:30
 0:07:43
0:07:43
 0:12:46
0:12:46
 0:03:41
0:03:41
 0:08:12
0:08:12
 0:16:27
0:16:27