filmov
tv
Single Replacement Reaction Practice Problems & Examples

Показать описание
👉 Support me on Patreon 👈
💻 My highly recommended chemistry resources
HIGH SCHOOL / GENERAL CHEMISTRY
ORGANIC CHEMISTRY
In this video, you'll learn how to predict the products of single replacement or single displacement reactions. We'll start with the basics of what single replacement reactions are and then go through three examples together step by step.
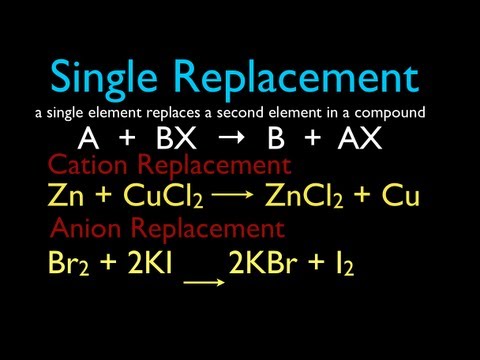
Single replacement reactions involves an ionic compound or ionic-like compounds, such as acids and bases, reacting with an element or compound composed of one element. The products are an ionic compound and element. In a single replacement reaction, the cations essentially switch place if the lone element carries a positive charge in ionic compound. The anions essentially switch place if the lone element carries a negative charge in ionic compound.
The best way to approach single replacement reactions is to first label the charges of each of the ions in the ionic compound and element.
By the end of this video, you'll know exactly how to predict the products of double replacement or double displacement reactions.
Single Replacement Reactions Practice Problems
Single Replacement Reaction Practice Problems & Examples
Predicting Products of Single Replacement Reactions
Predicting Products of Single Replacement Reactions
CLASSIFYING CHEMICAL REACTIONS - Single Replacement Sample Problem 1 - (slide 10)
Single Replacement Reaction || Easy Tutorial with Sir D.
Single Displacement Reactions - Problem Solving Session
Notes: Single Replacement Reactions
Predicting Products | Single Replacement Reactions
Plainfield Chemistry - Balancing Single Replacement & Double Replacement Reactions - Even Questi...
Classifying Types of Chemical Reactions Practice Problems
Chemical Reactions (2 of 11) Single Replacement Reactions, An Explanation
Single Replacement Reaction Example Problems with Beaker Diagrams
CLASSIFYING CHEMICAL REACTIONS - Single Replacement Sample Problem 2 - (slide 10)
Single Displacement Reactions: Activity Series Explained + 56 Examples !
Predicting Products of Chemical Reactions: Single Displacment
Single Replacement Reactions
Single Replacement Reactions
Double Replacement Reaction Practice Problems & Examples
Types of Chemical Reactions
Classifying Types of Chemical Reactions With Practice Problems | Study Chemistry With Us
Reaction Types: Single Replacement Reactions (2020)
How to predict products for single replacement reactions
Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems
Комментарии
 0:06:38
0:06:38
 0:05:59
0:05:59
 0:02:03
0:02:03
 0:10:18
0:10:18
 0:05:04
0:05:04
 0:05:39
0:05:39
 0:20:21
0:20:21
 0:08:23
0:08:23
 0:14:05
0:14:05
 0:18:33
0:18:33
 0:05:48
0:05:48
 0:09:28
0:09:28
 0:19:33
0:19:33
 0:03:05
0:03:05
 0:25:19
0:25:19
 0:06:28
0:06:28
 0:05:29
0:05:29
 0:10:37
0:10:37
 0:06:03
0:06:03
 0:40:06
0:40:06
 0:17:50
0:17:50
 0:23:08
0:23:08
 0:08:28
0:08:28
 0:18:42
0:18:42