filmov
tv
Predicting Products of Chemical Reactions: Single Displacment

Показать описание
In this video we will predict the products of a single displacement reaction using the activity series of metals.
Thanks for watching. Please 'like' and 'subscribe'
Thanks for watching. Please 'like' and 'subscribe'
Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems
How to Predict Products of Chemical Reactions | How to Pass Chemistry
Predicting the Products of Chemical Reactions
Solving Chemical Reactions - Predicting the Products - CLEAR & SIMPLE CHEMISTRY
How to Predict Products of Chemical Reactions ?
Predicting Products of Single Replacement Reactions
Predicting Products of Chemical Reactions: Single Displacment
Predicting Products of Chemical Reactions
Predicting Products of Single Replacement Reactions
Predicting Products of Chemical Reactions: Practice Problems
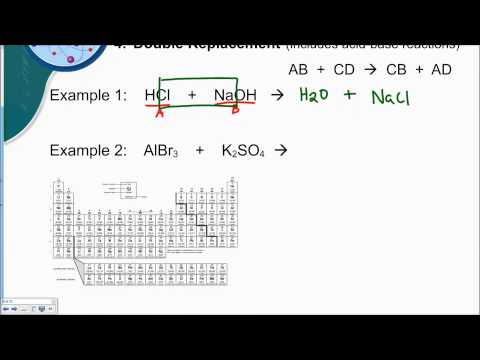
Predicting Products of Double Replacement Reactions
Types of Chemical Reactions
Types of Chemical Reactions
Synthesis Reactions: Predict the Products (100 Examples)
Predicting Products of Chemical Reactions
ALEKS: Predicting the products of a neutralization reaction
Chemical Reactions- Predicting Products
Predicting Products of Chemical Reactions Practice
Decomposition Reactions: Predicting Products
More Examples and Practice: How to Predict and Balance Chemical Reactions
Classifying Types of Chemical Reactions Practice Problems
Predicting Products | Decomposition Reactions
Types of Chemical Reactions
Predicting Products of Decomposition Reactions
Комментарии
 0:18:42
0:18:42
 0:04:50
0:04:50
 0:09:05
0:09:05
 0:07:39
0:07:39
 0:08:16
0:08:16
 0:02:03
0:02:03
 0:13:06
0:13:06
 0:10:18
0:10:18
 0:18:33
0:18:33
 0:02:49
0:02:49
 0:12:54
0:12:54
 0:40:06
0:40:06
 0:24:33
0:24:33
 0:23:03
0:23:03
 0:02:47
0:02:47
 0:11:15
0:11:15
 0:14:07
0:14:07
 0:10:01
0:10:01
 0:17:08
0:17:08
 0:05:48
0:05:48
 0:10:44
0:10:44
 0:03:00
0:03:00
 0:03:40
0:03:40