filmov
tv
Formula Writing Binary ionic Compounds Names to Formulas

Показать описание
Formula Writing Binary ionic Compounds Names to Formulas
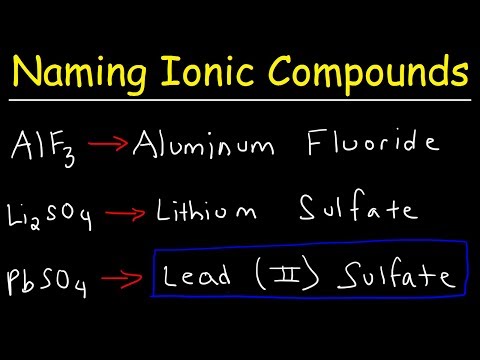
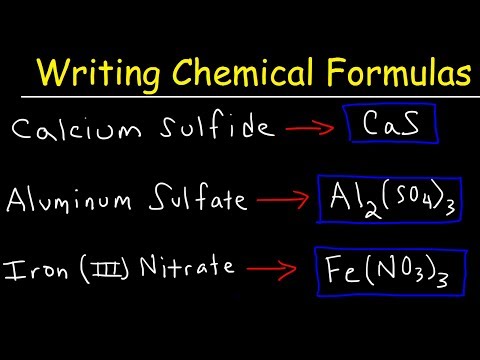
When given a chemical name for a binary ionic equation you should be able to create a proper formula using your Chemistry reference tables and the Periodic Table of elements.
In this case we have a metal and a non metal bonded together and will use oxidation numbers/states from the periodic table and the "criss cross" method of creating the proper formula which I show in this video.
I also provide examples of how to use the stock system and roman numerals to determine which oxidation number to correctly use. For example:
I = +1
II = +2
III = +3
IV = +4
Keywords:
formula writing and nomenclature, formula writing, naming binary compounds, chemistry reference table, periodic table of elements, oxidation numbers, criss cross method of naming compounds, ionic compounds,
See all Chemistry reactions, calculations and tutorials here:
When given a chemical name for a binary ionic equation you should be able to create a proper formula using your Chemistry reference tables and the Periodic Table of elements.
In this case we have a metal and a non metal bonded together and will use oxidation numbers/states from the periodic table and the "criss cross" method of creating the proper formula which I show in this video.
I also provide examples of how to use the stock system and roman numerals to determine which oxidation number to correctly use. For example:
I = +1
II = +2
III = +3
IV = +4
Keywords:
formula writing and nomenclature, formula writing, naming binary compounds, chemistry reference table, periodic table of elements, oxidation numbers, criss cross method of naming compounds, ionic compounds,
See all Chemistry reactions, calculations and tutorials here:
 0:10:22
0:10:22
 0:11:44
0:11:44
 0:05:44
0:05:44
 0:12:04
0:12:04
 0:10:45
0:10:45
 0:11:09
0:11:09
 0:07:13
0:07:13
 0:10:09
0:10:09
 0:04:39
0:04:39
 0:01:30
0:01:30
 0:16:00
0:16:00
 0:00:53
0:00:53
 0:14:20
0:14:20
 0:05:40
0:05:40
 0:05:30
0:05:30
 0:13:33
0:13:33
 0:13:29
0:13:29
 0:17:56
0:17:56
 0:10:32
0:10:32
 0:04:32
0:04:32
 0:15:22
0:15:22
 0:10:41
0:10:41
 0:09:02
0:09:02
 0:10:10
0:10:10