filmov
tv
Type II Binary Ionic Compounds - Naming and Writing Formulas

Показать описание
In this video we will learn about type II binary ionic compounds and learn how to name and write the chemical formulas for them. We will also work out several examples and at the very end students will be given a few to try on their own.
Type II Binary Ionic Compounds - Naming and Writing Formulas
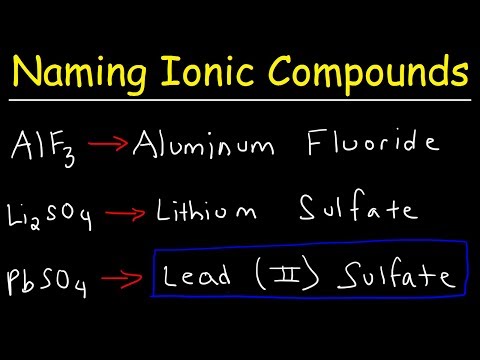
Naming Type II Binary Ionic Compounds
Naming Type II Binary Ionic Compounds
Naming Rules for Binary Ionic Compounds
HOW TO NAME TYPE II IONIC COMPOUNDS
Naming Ionic Compounds (Type II)
Writing Formulas for Type II Binary Ionic Compounds
Type II Binary Ionic Compounds Lecture Video
Nomenclature: Binary ionic compounds (Type II)
Naming ionic compounds with variable charges Type II Binary compounds
Binary Ionic Compounds
Naming Ionic Compounds (Type 1, 2, & 3)
How To Name Ionic Compounds With Transition Metals
Writing Ionic Formulas: Introduction
Type I Binary Ionic Compounds - Naming and Writing Formulas
Naming Ionic Compounds
Naming Ionic and Molecular Compounds | How to Pass Chemistry
Writing Formulas For Binary Ionic Compounds - Practice - 2
Binary Ionic Compounds Nomenclature
4.1 Naming Type 2 Binary Ionic Compounds
Type II Ionic Compounds
Type II Ionic Compounds
GCSE Chemistry - What is an Ionic Compound? Ionic Compounds Explained #15
HOW TO WRITE FORMULAS FOR TYPE II IONIC COMPOUNDS
Комментарии
 0:13:29
0:13:29
 0:10:00
0:10:00
 0:08:39
0:08:39
 0:01:30
0:01:30
 0:08:15
0:08:15
 0:04:35
0:04:35
 0:07:18
0:07:18
 0:19:26
0:19:26
 0:05:17
0:05:17
 0:08:58
0:08:58
 0:05:40
0:05:40
 0:20:40
0:20:40
 0:13:33
0:13:33
 0:11:44
0:11:44
 0:10:45
0:10:45
 0:05:44
0:05:44
 0:10:32
0:10:32
 0:09:02
0:09:02
 0:15:56
0:15:56
 0:10:10
0:10:10
 0:11:21
0:11:21
 0:08:21
0:08:21
 0:06:08
0:06:08
 0:07:17
0:07:17