filmov
tv
What Makes SF4 act Like a Lewis Base and Lewis Base in Certain Situations? | Hybridization

Показать описание
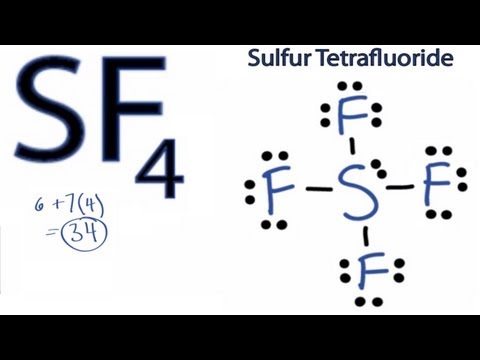
SF4 can acts as Lewis Acid in the presence
of strong Lewis Base and also acts as Lewis Base in the presence of strong Lewis Acid.
Lewis acid: Accepts electrons
Lewis base: Donates electrons
Watch this video to know details why SF4 can behaves as Lewis acid and Lewis base.
Hi! I'm Dr. Anuradha Mukherjee from Chemistry Affinity. I have designed this you tube channel to help you in your journey to learn organic chemistry.
I was the chemistry educator of BS (Research) programe in IISc, Bangalore. I am an chemistry educator and academic consultant. My dream and aim is to teach students chemistry in fascinating way so that their chemistry concepts become clear. They Strat to love this subject. Then it will be easy for them in exam preparation.
I have published a book on Green Chemistry.
Check my LinkedIn profile
Check my Chemistry Affinity Facebook page
Do you want to suggest an idea for the next chemistry video? Send me a message to my email
Reaction Map -1
Reaction Map -2
Reaction Map -3
Reaction map-4
Reaction map -5
Reaction map-6
Reaction map-7
Reaction map-8
Reaction map-9
Reaction map-10
Reaction map-11
Reaction map-12
of strong Lewis Base and also acts as Lewis Base in the presence of strong Lewis Acid.
Lewis acid: Accepts electrons
Lewis base: Donates electrons
Watch this video to know details why SF4 can behaves as Lewis acid and Lewis base.
Hi! I'm Dr. Anuradha Mukherjee from Chemistry Affinity. I have designed this you tube channel to help you in your journey to learn organic chemistry.
I was the chemistry educator of BS (Research) programe in IISc, Bangalore. I am an chemistry educator and academic consultant. My dream and aim is to teach students chemistry in fascinating way so that their chemistry concepts become clear. They Strat to love this subject. Then it will be easy for them in exam preparation.
I have published a book on Green Chemistry.
Check my LinkedIn profile
Check my Chemistry Affinity Facebook page
Do you want to suggest an idea for the next chemistry video? Send me a message to my email
Reaction Map -1
Reaction Map -2
Reaction Map -3
Reaction map-4
Reaction map -5
Reaction map-6
Reaction map-7
Reaction map-8
Reaction map-9
Reaction map-10
Reaction map-11
Reaction map-12
 0:02:08
0:02:08
 0:06:23
0:06:23
 0:01:49
0:01:49
 0:00:06
0:00:06
 0:03:19
0:03:19
 0:08:36
0:08:36
 0:00:11
0:00:11
 0:03:48
0:03:48
 0:00:21
0:00:21
 0:00:59
0:00:59
 0:00:53
0:00:53
 0:00:51
0:00:51
 0:00:23
0:00:23
 0:00:20
0:00:20
 0:00:41
0:00:41
 0:00:57
0:00:57
 0:01:49
0:01:49
 0:00:07
0:00:07
 0:04:30
0:04:30
 0:00:53
0:00:53
 0:00:59
0:00:59
 0:00:09
0:00:09
 0:07:52
0:07:52
 0:01:54
0:01:54