filmov
tv
Part 1: Effective Nuclear Charge (Slater's Rule) for IIT JAM/NEET
Показать описание
Note: For Cr atom the problem solved is wrong, kindly ignore, I have uploaded a correction of the problem. For an atom or an ion with only a single electron, we can calculate the potential energy by considering only the electrostatic attraction between the positively charged nucleus and the negatively charged electron. When more than one electron is present, however, the total energy of the atom or the ion depends not only on attractive electron-nucleus interactions but also on repulsive electron-electron interactions. When there are two electrons, the repulsive interactions depend on the positions of both electrons at a given instant, but because we cannot specify the exact positions of the electrons, it is impossible to exactly calculate the repulsive interactions. Consequently, we must use approximate methods to deal with the effect of electron-electron repulsions on orbital energies. These effects are the underlying basis for the periodic trends in elemental properties that we will explore in this chapter.
Electron Shielding and Effective Nuclear Charge
If an electron is far from the nucleus (i.e., if the distance r
between the nucleus and the electron is large), then at any given moment, many of the other electrons will be between that electron and the nucleus (Figure 7.2.1). Hence the electrons will cancel a portion of the positive charge of the nucleus and thereby decrease the attractive interaction between it and the electron farther away. As a result, the electron farther away experiences an effective nuclear charge (Zeff) that is less than the actual nuclear charge Z. This effect is called electron shielding.
As the distance between an electron and the nucleus approaches infinity, Zeff approaches a value of 1 because all the other (Z−1) electrons in the neutral atom are, on the average, between it and the nucleus. If, on the other hand, an electron is very close to the nucleus, then at any given moment most of the other electrons are farther from the nucleus and do not shield the nuclear charge. At r≈0, the positive charge experienced by an electron is approximately the full nuclear charge, or Zeff≈Z. At intermediate values of r, the effective nuclear charge is somewhere between 1 and Z
1≤Zeff≤Z.(7.2.1)
Notice that Zeff=Zonly for hydrogen and only for helium are Zeff and Z comparable in magnitude
Shielding
Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Hence, the nucleus has "less grip" on the outer electrons insofar as it is shielded from them.Zeff
can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation:
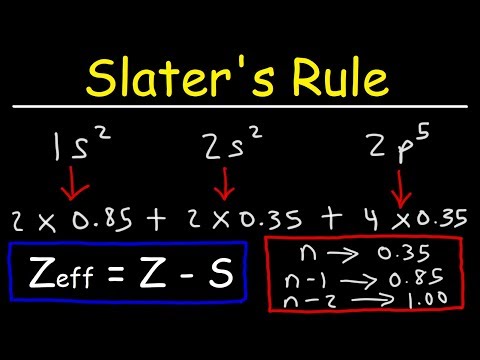
Zeff=Z−S(7.2.2)
where Z
is the atomic number (number of protons in nucleus) and S is the shielding constant. The value of Zeff will provide information on how much of a charge an electron actually experiences.
We can see from Equation 7.2.2 that the effective nuclear charge of an atom increases as the number of protons in an atom increases. Therefore as we go from left to right on the periodic table the effective nuclear charge of an atom increases in strength and holds the outer electrons closer and tighter to the nucleus. As we will discuss later on in the chapter, this phenomenon can explain the decrease in atomic radii we see as we go across the periodic table as electrons are held closer to the nucleus due to increase in number of protons and increase in effective nuclear charge.The shielding constant can be estimated by totaling the screening by all electrons (n) except the one in question.
S=∑in−1Si(7.2.3)
where Si is the shielding of the ith electron.
Electrons that are shielded from the full charge of the nucleus experience an effective nuclear charge (Zeff ) of the nucleus, which is some degree less than the full nuclear charge an electron would feel in a hydrogen atom or hydrogenlike ion.From Equations 7.2.2and 7.2.3, Zeff
for a specific electron can be estimated is the shielding constants for that electron of all other electrons in species is known. A simple approximation is that all other electrons shield equally and fully:
Si=1
Electron Shielding and Effective Nuclear Charge
If an electron is far from the nucleus (i.e., if the distance r
between the nucleus and the electron is large), then at any given moment, many of the other electrons will be between that electron and the nucleus (Figure 7.2.1). Hence the electrons will cancel a portion of the positive charge of the nucleus and thereby decrease the attractive interaction between it and the electron farther away. As a result, the electron farther away experiences an effective nuclear charge (Zeff) that is less than the actual nuclear charge Z. This effect is called electron shielding.
As the distance between an electron and the nucleus approaches infinity, Zeff approaches a value of 1 because all the other (Z−1) electrons in the neutral atom are, on the average, between it and the nucleus. If, on the other hand, an electron is very close to the nucleus, then at any given moment most of the other electrons are farther from the nucleus and do not shield the nuclear charge. At r≈0, the positive charge experienced by an electron is approximately the full nuclear charge, or Zeff≈Z. At intermediate values of r, the effective nuclear charge is somewhere between 1 and Z
1≤Zeff≤Z.(7.2.1)
Notice that Zeff=Zonly for hydrogen and only for helium are Zeff and Z comparable in magnitude
Shielding
Shielding refers to the core electrons repelling the outer electrons, which lowers the effective charge of the nucleus on the outer electrons. Hence, the nucleus has "less grip" on the outer electrons insofar as it is shielded from them.Zeff
can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the equation:
Zeff=Z−S(7.2.2)
where Z
is the atomic number (number of protons in nucleus) and S is the shielding constant. The value of Zeff will provide information on how much of a charge an electron actually experiences.
We can see from Equation 7.2.2 that the effective nuclear charge of an atom increases as the number of protons in an atom increases. Therefore as we go from left to right on the periodic table the effective nuclear charge of an atom increases in strength and holds the outer electrons closer and tighter to the nucleus. As we will discuss later on in the chapter, this phenomenon can explain the decrease in atomic radii we see as we go across the periodic table as electrons are held closer to the nucleus due to increase in number of protons and increase in effective nuclear charge.The shielding constant can be estimated by totaling the screening by all electrons (n) except the one in question.
S=∑in−1Si(7.2.3)
where Si is the shielding of the ith electron.
Electrons that are shielded from the full charge of the nucleus experience an effective nuclear charge (Zeff ) of the nucleus, which is some degree less than the full nuclear charge an electron would feel in a hydrogen atom or hydrogenlike ion.From Equations 7.2.2and 7.2.3, Zeff
for a specific electron can be estimated is the shielding constants for that electron of all other electrons in species is known. A simple approximation is that all other electrons shield equally and fully:
Si=1
Комментарии
 0:07:14
0:07:14
 0:09:50
0:09:50
 0:01:47
0:01:47
 0:04:47
0:04:47
 0:11:40
0:11:40
 0:12:30
0:12:30
 0:17:27
0:17:27
 0:07:32
0:07:32
 1:11:42
1:11:42
 0:22:31
0:22:31
 0:02:58
0:02:58
 0:07:53
0:07:53
 0:10:50
0:10:50
 0:03:05
0:03:05
 0:00:48
0:00:48
 0:05:25
0:05:25
 0:07:17
0:07:17
 0:13:59
0:13:59
 0:09:31
0:09:31
 0:08:05
0:08:05
 1:59:33
1:59:33
 0:09:01
0:09:01
 0:15:18
0:15:18
 0:04:47
0:04:47